Metopirone
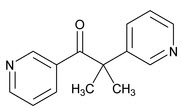
What is Metopirone (Metyrapone)?
Approved To Treat
Related Clinical Trials
Summary: The investigator aim to understand whether food-induced glucocorticoids influence fat mass in overweight and obese people. In a randomized, cross-over study, 23 overweight and obese volunteers will receive a block and replace therapy that mimics physiological glucocorticoid (GC) rhythm (metyrapone plus hydrocortisone) or placebo. Participants will undergo two identical overfeeding periods with eac...
Summary: This study is a national, multicenter, interventional, phase II clinical trial on the use of pembrolizumab in advanced adrenocortical carcinoma, with confirmed progression within 6 months, following EDP or EDP-M ( etoposide, doxorubicin, cisplatin- mitotan) chemotherapy. Adrenocortical carcinoma is a very rare entity with poor prognosis and limited therapeutic options. Only radical surgical treatm...
Summary: Drug interventional, controlled, randomized open-label, parallel-group, multicenter study in patients with bilateral adrenal incidentalomas associated with subclinical Cushing's syndrome
Related Latest Advances
Brand Information
- Cardiovascular System:Hypotension
- Gastrointestinal System:Nausea, vomiting, abdominal discomfort or pain
- Central Nervous System:Headache, dizziness, sedation
- Dermatologic System:Allergic rash
- Hematologic System:Leukopenia, anemia, and/or thrombocytopenia