Brand Name
Aczone
Generic Name
Dapsone
View Brand Information FDA approval date: June 24, 2009
Classification: Sulfone
Form: Tablet, Gel
What is Aczone (Dapsone)?
Dapsone gel, 5%, is indicated for the topical treatment of acne vulgaris. Dapsone gel, 5% is indicated for the topical treatment of acne vulgaris.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Rab 32 Gene Polymorphisms as a Prognostic Factor in Leprosy Patients and Its Relation to Multiple Drug Therapy: Randomized Clinical Trial
Summary: Mycobacterium leprae is a slow-growing bacillus that causes leprosy. the infection may take two to ten years to incubate. While the exact mechanism of infection transmission is unknown, direct bacillus absorption through the nasal or respiratory mucosa and aerosolized nasal secretions are the most common theories. The bacteria is subsequently transported by the bloodstream to the peripheral nerves...
A Randomized Multicenter Study for Isolated Skin Vasculitis
Summary: Multi-center sequential multiple assignment randomized trial comparing the effectiveness of three different standard of care treatment options for patients with isolated skin vasculitis.
Related Latest Advances
Brand Information
Aczone (dapsone)
1INDICATIONS AND USAGE
ACZONE
2DOSAGE AND ADMINISTRATION
For topical use only. Not for oral, ophthalmic, or intravaginal use.
After the skin is gently washed and patted dry, apply approximately a pea-sized amount of ACZONE Gel, 7.5%, in a thin layer to the entire face once daily. In addition, a thin layer may be applied to other affected areas once daily. Rub in ACZONE Gel, 7.5%, gently and completely.
If there is no improvement after 12 weeks, treatment with ACZONE Gel, 7.5% should be reassessed.
3DOSAGE FORMS AND STRENGTHS
Gel, 7.5%. Each gram of ACZONE Gel, 7.5% contains 75 mg of dapsone in an off-white to yellow gel with suspended particles.
4CONTRAINDICATIONS
None.
5DRUG INTERACTIONS
No formal drug-drug interaction studies were conducted with ACZONE Gel, 7.5%.
5.1Trimethoprim-Sulfamethoxazole
A drug-drug interaction study evaluated the effect of the use of dapsone gel, 5% in combination with double strength (160 mg/800 mg) trimethoprim-sulfamethoxazole (TMP/SMX). During co-administration, systemic levels of TMP and SMX were essentially unchanged, however, levels of dapsone and its metabolites increased in the presence of TMP/SMX. The systemic exposure from ACZONE Gel, 7.5% is expected to be about 1% of that from the 100 mg oral dose, even when co-administered with TMP/SMX.
5.2Topical Benzoyl Peroxide
Topical application of dapsone gel followed by benzoyl peroxide in patients with acne vulgaris may result in a temporary local yellow or orange discoloration of the skin and facial hair.
5.3Drug Interactions with Oral Dapsone
Certain concomitant medications (such as rifampin, anticonvulsants, St. John’s wort) may increase the formation of dapsone hydroxylamine, a metabolite of dapsone associated with hemolysis. With oral dapsone treatment, folic acid antagonists such as pyrimethamine have been noted to possibly increase the likelihood of hematologic reactions.
5.4Concomitant Use with Drugs that Induce Methemoglobinemia
Concomitant use of ACZONE Gel, 7.5% with drugs that induce methemoglobinemia such as sulfonamides, acetaminophen, acetanilide, aniline dyes, benzocaine, chloroquine, dapsone, naphthalene, nitrates and nitrites, nitrofurantoin, nitroglycerin, nitroprusside, pamaquine, para‐aminosalicylic acid, phenacetin, phenobarbital, phenytoin, primaquine, and quinine may increase the risk for developing methemoglobinemia
6DESCRIPTION
ACZONE (dapsone) Gel, 7.5%, contains dapsone, a sulfone, in an aqueous gel base for topical dermatologic use. ACZONE Gel, 7.5% is an off-white to yellow gel with suspended particles. Chemically, dapsone has an empirical formula of C
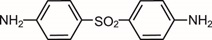
Each gram of ACZONE Gel, 7.5%, contains 75 mg of dapsone, USP, in a gel of diethylene glycol monoethyl ether, methylparaben, acrylamide/sodium acryloyldimethyl taurate copolymer, isohexadecane, polysorbate 80, and purified water.
7CLINICAL STUDIES
The safety and efficacy of once daily use of ACZONE Gel, 7.5%, was assessed in two 12-week multicenter, randomized, double-blind, vehicle-controlled trials. Efficacy was assessed in a total of 4340 subjects 12 years of age and older. The majority of the subjects had moderate acne vulgaris, 20 to 50 inflammatory and 30 to 100 non-inflammatory lesions at baseline, and were randomized to receive either ACZONE Gel, 7.5% or vehicle.
Treatment response was defined at Week 12 as the proportion of subjects who were rated “none” or “minimal” with at least a two-grade improvement from baseline on the Global Acne Assessment Score (GAAS), and mean absolute change from baseline in both inflammatory and non-inflammatory lesion counts. A GAAS score of “none” corresponded to no evidence of facial acne vulgaris. A GAAS score of “minimal” corresponded to a few non-inflammatory lesions (comedones) being present and to a few inflammatory lesions (papules/pustules) that may be present.
The GAAS success rate, mean reduction, and percent reduction in acne lesion counts from baseline after 12 weeks of treatment are presented in the following table.
8HOW SUPPLIED/STORAGE AND HANDLING
ACZONE Gel is an off-white to yellow gel with suspended particles. It is supplied in an airless pump containing a polypropylene bottle with a high density polyethylene piston.
ACZONE (dapsone) Gel, 7.5%, is supplied in the following sizes:
NDC 16110-526-30 30 gram pump
NDC 16110-526-60 60 gram pump
NDC 16110-526-90 90 gram pump
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Hematological Effects
- Inform patients that methemoglobinemia can occur with topical dapsone treatment. Advise patients to seek immediate medical attention if they develop cyanosis
- Inform patients who have G6PD deficiency that hemolytic anemia may occur with topical dapsone treatment. Advise patients to seek medical attention if they develop signs and symptoms suggestive of hemolytic anemia
Important Administration Instructions
- Advise patients to apply ACZONE Gel, 7.5%, once daily to the entire face
- ACZONE Gel, 7.5% is for topical use only.
- Do not apply ACZONE Gel, 7.5% to eyes, mouth, or mucous membranes.
Distributed by: Almirall, LLC.
Malvern, PA 19355
Aczone
© 2019 Almirall, LLC. All rights reserved.
10PRINCIPAL DISPLAY PANEL - NDC: 16110-526-60 - 60 g Pump Label

11PRINCIPAL DISPLAY PANEL - NDC: 16110-526-60 - 60 g Carton Label
12PRINCIPAL DISPLAY PANEL - NDC: 16110-526-90 - 90 g Pump Label

13PRINCIPAL DISPLAY PANEL - NDC: 16110-526-90 - 90 g Carton Label



