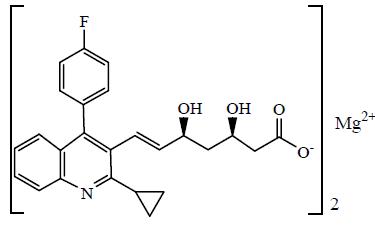
Pitavastatin
What is Zypitamag (Pitavastatin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a randomized, investigator-and-participant-blind, placebo-controlled, fixed sequence, cross-over, Phase 1 study to investigate the safety, tolerability, and pharmacokinetics of multiple doses of orally administered RO7795081 and the effect of a steady-state dose of orally administered RO7795081 on the pharmacokinetics of pitavastatin and rosuvastatin in otherwise healthy, overweight or obe...
Summary: People with HIV are at a higher risk of cardiovascular diseases (CVD) due to the effects of the virus and its treatment. Integrase strand transfer inhibitors (INSTIs), a common HIV treatment, are associated with increased CVD risk and metabolic issues, such as weight gain and high blood pressure. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, however, have been working well in reducing CVD eve...
Summary: A Multicenter, randomized trial comparing the efficacy and safety of intensive lipid-lowering therapy using a statin-ezetimibe combination without aspirin versus statin monotherapy with aspirin in asymptomatic patients with coronary artery calcification
Related Latest Advances
Brand Information
- Concomitant use of cyclosporine
- Acute liver failure or decompensated cirrhosis
- Hypersensitivity to pitavastatin or any excipients in ZYPITAMAG. Hypersensitivity reactions including angioedema, rash, pruritus, and urticaria have been reported with pitavastatin
- Myopathy and Rhabdomyolysis
- Immune-Mediated Necrotizing Myopathy
- Hepatic Dysfunction
- Increases in HbA1c and Fasting Serum Glucose Levels
In 10 controlled clinical studies and 4 subsequent open-label extension studies, 3,291 adult patients with primary hyperlipidemia were administered pitavastatin 1 mg to 4 mg daily. The mean continuous exposure of pitavastatin (1 mg to 4 mg) was 36.7 weeks (median 51.1 weeks). The mean age of the patients was 60.9 years (range; 18 years – 89 years) and 52% females. Approximately 93% of the patients were White 7% were Asian/Indian, 0.2% were African American and 0.3% were Hispanic and other.
Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Protect from moisture and light.
Inform patients that ZYPITAMAG may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (.
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with ZYPITAMAG. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (.
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if ZYPITAMAG should be discontinued [see Use in Specific Populations (
Advise patients that breastfeeding is not recommended during treatment with ZYPITAMAG [see Use in Specific Populations (.
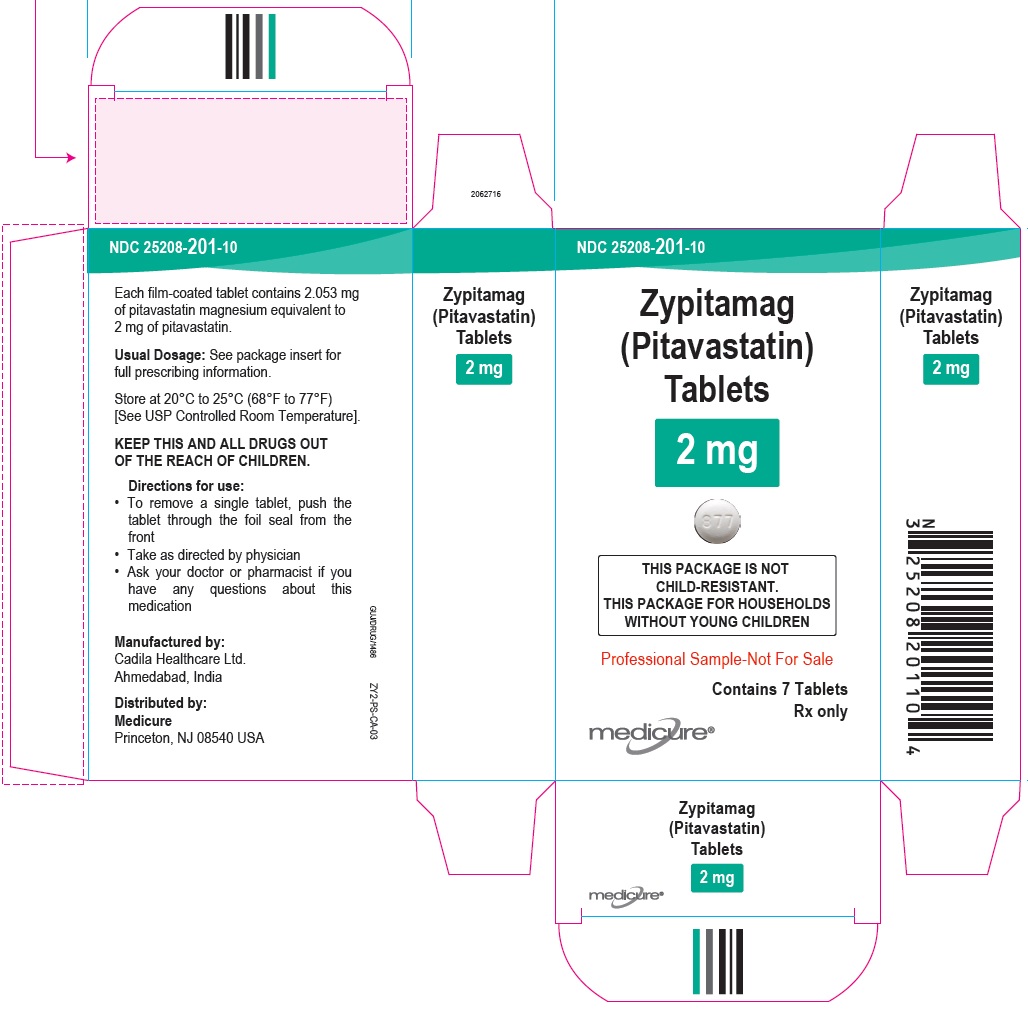
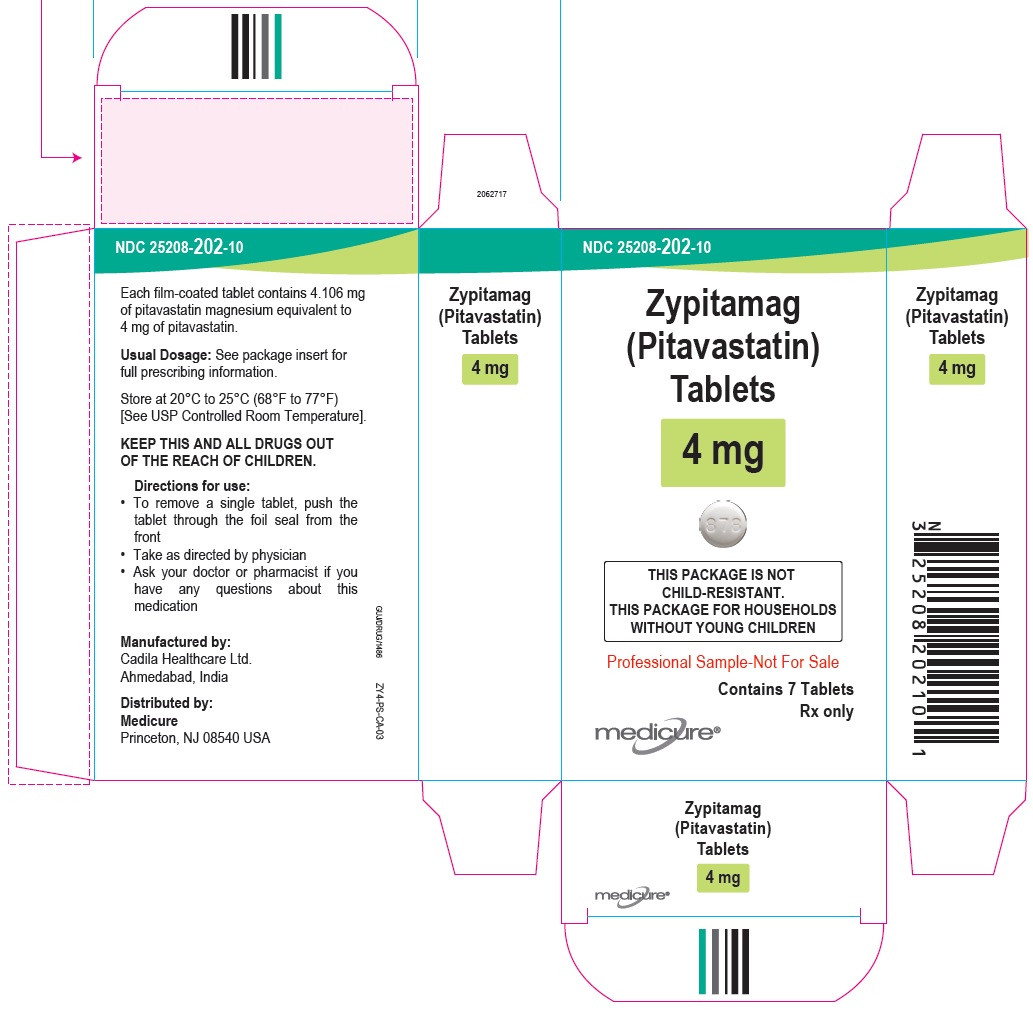
NDC 25208-201-09