Valtrex
What is Valtrex (ValACYclovir)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: To learn if the combination of pirtobrutinib (also called LOXO-305), venetoclax, and obinutuzumab is safe and effective when given to patients with chronic lymphocytic leukemia (CLL) or Richter transformation (RT) who have not previously received treatment.
Summary: Congenital cytomegalovirus (CMV) infection is the most common congenital infection with a birth prevalence of 0.4% in Europe. It is the leading non-genetic cause of sensorineural hearing loss and a major cause of neurodevelopmental disabilities. The risk of intrauterine transmission is highest when primary infection occurs during pregnancy. Primary CMV infection is asymptomatic or causes non-speci...
Summary: The Virus in Endodontics (VE) phase I pilot study for preoperative pain will be analyzed and adjusted for the Phase II clinical trial. The Phase II clinical trial on preoperative pain, postoperative pain, and clinical healing will involve 250 patients. Patients will be recruited from the same pool of participants as the Phase II clinical trial. Preoperative cone beam computed tomography (CBCT) sca...
Related Latest Advances
Brand Information
- VALTREX may be given without regard to meals.
- Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) may be prepared extemporaneously from 500-mg VALTREX tablets for use in pediatric patients for whom a solid dosage form is not appropriate
- Prepare SSV according to the USP-NF.
- Using a pestle and mortar, grind the required number of VALTREX 500-mg tablets until a fine powder is produced (5 VALTREX tablets for 25-mg/mL suspension; 10 VALTREX tablets for 50-mg/mL suspension).
- Gradually add approximately 5-mL aliquots of SSV to the mortar and triturate the powder until a paste has been produced. Ensure that the powder has been adequately wetted.
- Continue to add approximately 5-mL aliquots of SSV to the mortar, mixing thoroughly between additions, until a concentrated suspension is produced, to a minimum total quantity of 20 mL SSV and a maximum total quantity of 40 mL SSV for both the 25-mg/mL and 50-mg/mL suspensions.
- Transfer the mixture to a suitable 100-mL measuring flask.
- Transfer the cherry flavor* to the mortar and dissolve in approximately 5 mL of SSV. Once dissolved, add to the measuring flask.
- Rinse the mortar at least 3 times with approximately 5-mL aliquots of SSV, transferring the rinsing to the measuring flask between additions.
- Make the suspension to volume (100 mL) with SSV and shake thoroughly to mix.
- Transfer the suspension to an amber glass medicine bottle with a child-resistant closure.
- The prepared suspension should be labeled with the following information “Shake well before using. Store suspension between 2°C to 8°C (36°F to 46°F) in a refrigerator. Discard after 28 days.”
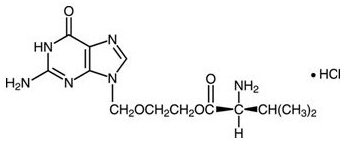
- 500-mg: Each blue, film‑coated, capsule‑shaped tablet printed with “VALTREX 500 mg” contains 556.2 mg of valacyclovir hydrochloride equivalent to 500 mg of the free base.
- 1-gram: Each blue, film‑coated, capsule‑shaped tablet, with a partial scorebar on both sides, printed with “VALTREX 1 gram” contains 1.112 grams of valacyclovir hydrochloride equivalent to 1 gram of the free base.
- Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome
- Acute Renal Failure
- Central Nervous System Effects