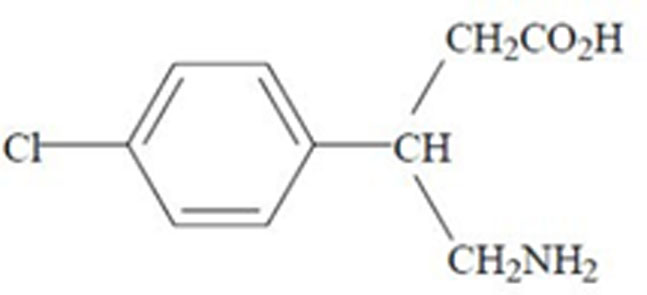
Baclofen
What is Lyvispah (Baclofen)?
Related Clinical Trials
Summary: The goal of this clinical trial is to assess the effect of using baclofen along with conventional treatment in improving GERD symptoms. It will also assess the safety of drug baclofen by recording the patient reported adverse events. The main questions it aims to answer are : Does drug baclofen along with conventional treatment has any effect on patients with GERD symptoms? What medical problems d...
Summary: The goal of this clinical trial is to better understand the effects of intrathecal baclofen (ITB) on children with dystonic cerebral palsy (CP). The main questions this study aims to answer are: (1) Determine if ITB reduces dystonia while identifying other potential benefits, (2) Identify the characteristics of children with the best response to ITB (3) Develop a holistically representative compos...
Summary: The fight against alcoholism is a public health priority. Around 15 million Europeans and 10 million North Americans are alcohol dependent. Worldwide, 1 death out of 25 is thought to be attributed to alcohol. In France, the latest published data on alcohol-related mortality indicates that there were 49,000 alcohol-related deaths in 2009. Alcohol is thought to be the leading cause of hospitalisatio...
Related Latest Advances
Brand Information
- Adverse Reactions from Abrupt Withdrawal of LYVISPAH
- Neonatal Withdrawal Symptoms
- Drowsiness and Sedation
- Poor Tolerability in Stroke Patients
- Exacerbation of Psychotic Disorders, Schizophrenia, or Confusional States
- Exacerbation of Autonomic Dysreflexia
- Exacerbation of Epilepsy
- Posture and Balance Effects
- Ovarian Cysts
- Flush the feeding tube with up to 15 mL (one tablespoonful) of water.
- Open and empty the full contents of one packet of LYVISPAH in 15 mL (one tablespoonful) of the preferred liquid. Stir the suspension to ensure all granules are wetted.
- Draw up the suspension of granules into a dosing syringe immediately after stirring and administer the dose via the feeding tube. Administer no longer than two hours after mixing.
- Refill the dosing syringe with 15 mL (one tablespoonful) of water and flush the feeding tube with the remaining contents.
- Any unused suspensions should be discarded.

- Open 1 packet of LYVISPAH (see the figure above).
- Empty the full contents of the LYVISPAH packet into 1 tablespoon (15 mL) liquid or soft food and mix it.
- Take LYVISPAH within 2 hours of mixing into liquids or soft foods.
- If more than 1 packet of LYVISPAH is needed for your prescribed dose, mix each packet with a separate amount of liquid or soft food.
- Flush the feeding tube with up to 1 tablespoon (15 mL) of water using a catheter tip syringe.
- Open and empty the full contents of 1 packet of LYVISPAH into a clean container and mix with 1 tablespoon (15 mL) of liquid (apple juice or milk).
- Stir the mixture to make sure all the granules are wet.
- Draw up the mixture of granules into a dosing syringe right away after stirring.
- Give the dose of LYVISPAH through the feeding tube within 2 hours after mixing. If the mixture is in the dosing syringe for 15 minutes and not given, turn the dosing syringe upside down 3 times before you give the dose.
- Fill the dosing syringe with 1 tablespoon (15 mL) of water and flush the feeding tube.
- If more than 1 packet of LYVISPAH is needed for the prescribed dose, mix each packet with a separate amount of liquid.
- Throw away (dispose of) any unused mixture.


