Tazobactam
What is Piperacillin (Tazobactam)?
Approved To Treat
Related Clinical Trials
Summary: Data regarding optimal treatment for extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae blood-stream infection are lacking. Observational studies show conflicting results when comparing treatment with combination beta-lactam-beta-lactamase inhibitor and carbapenems. The investigators aim to evaluate the effect of definitive treatment with meropenem vs. piperacillin-tazobactam on ...
Summary: The EMPRESS trial aims to test the two most commonly used antibiotics (meropenem and piperacillin/tazobactam) among intensive care patients with sepsis (blood poisoning), as the safety of these two drugs is unclear in this group of patients.
Summary: Pneumonia is characterized by high incidence and mortality rates in elderly patients (≥65 years old). Piperacillin-tazobactam, as a broad-spectrum antibiotic, has its therapeutic efficacy and safety potentially influenced by the infusion regimen. However, research on infusion regimens specifically targeting the elderly population is currently limited. In our preliminary dose simulation, under the ...
Related Latest Advances
Brand Information
- Hypersensitivity Adverse Reactions
- Severe Cutaneous Adverse Reactions
- Hemophagocytic Lymphohistiocytosis
- Rhabdomyolysis [
- Hematologic Adverse Reactions
- Central Nervous System Adverse Reactions
- Nephrotoxicity in Critically Ill Patients
- Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.9)]
During the initial clinical investigations, 2621 patients worldwide were treated with piperacillin and tazobactam in phase 3 trials. In the key North American monotherapy clinical trials (n=830 patients), 90% of the adverse events reported were mild to moderate in severity and transient in nature. However, in 3.2% of the patients treated worldwide, piperacillin and tazobactam was discontinued because of adverse events primarily involving the skin (1.3%), including rash and pruritus; the gastrointestinal system (0.9%), including diarrhea, nausea, and vomiting; and allergic reactions (0.5%).
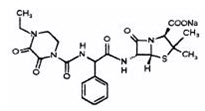
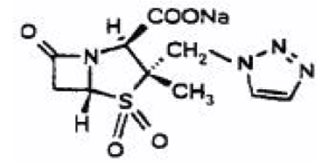
- Pharmacy Bulk Bottle
- Each pharmacy bulk package bottle provides piperacillin sodium equivalent to 36 grams of piperacillin and tazobactam sodium equivalent to 4.5 grams of tazobactam. Each pharmacy bulk package bottle contains 84.6 mEq (1,945 mg) of sodium.
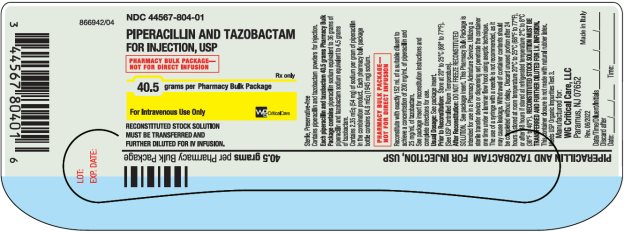
PIPERACILLIN AND TAZOBACTAM
Pharmacy Bulk Package-
Rx only




