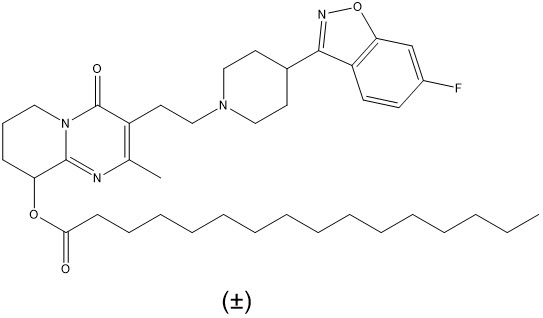
Paliperidone
What is Erzofri (Paliperidone)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The effectiveness and efficacy of the combination of pharmacotherapy with the two new recovery-oriented programs, RECOVERYTRSGR for patients with treatment- resistant schizophrenia and RECOVERYTRSBDGR for patients with treatment- resistant bipolar disorder.
Summary: The study wishes to examine whether extended antipsychotic treatment, in this case, antipsychotic treatment every other day, is as effective as daily treatment. It is also evaluating whether there may be differences in terms of side effects. Participants will be randomly assigned to either the treatment as usual group (i.e., taking antipsychotic daily) or the extended dosing group (i.e., taking an...
Summary: This prospective longitudinal cohort study will follow patients with schizophrenia who are treated with second generation long-acting injectable antipsychotic medications (LAIs) for 48 weeks to determine the risk of psychotic symptom relapse when treatment adherence is established. The study is designed to minimize the other factors that have contributed to breakthrough psychotic symptoms in patie...
Related Latest Advances
Brand Information
- Schizophrenia in adults
- Schizoaffective disorder in adults as monotherapy and as an adjunct to mood stabilizers or antidepressants
- Increased mortality in elderly patients with dementia-related psychosis
- Cerebrovascular adverse reactions, including stroke, in elderly patients with dementia-related psychosis
- Neuroleptic malignant syndrome
- QT prolongation
- Tardive dyskinesia
- Metabolic changes
- Orthostatic hypotension and syncope
- Falls
- Leukopenia, neutropenia, and agranulocytosis
- Hyperprolactinemia
- Potential for cognitive and motor impairment
- Seizures
- Dysphagia
- Priapism
- Disruption of body temperature regulation