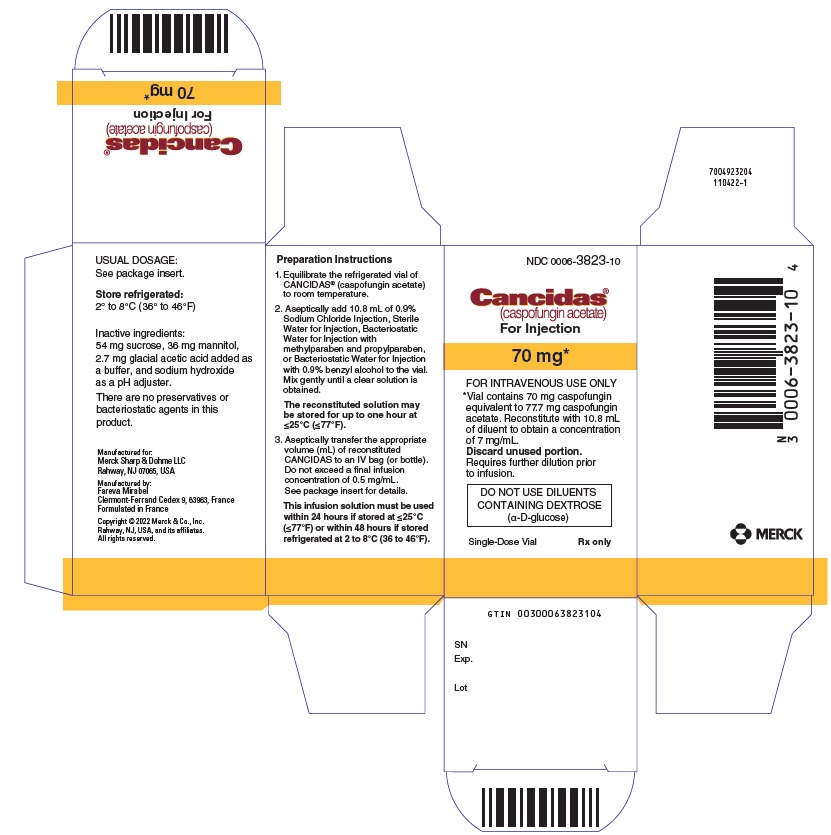
Cancidas
What is Cancidas (Caspofungin Acetate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this clinical trial is to learn about the safety and effects of the study medicine (called Fosmanogepix) for the potential treatment of candidemia and/or invasive candidiasis, a life-threatening fungal infection caused by several species of yeast called Candida. The study is seeking patients who have a diagnosis of candidemia and/or invasive candidiasis. Two-thirds of all patients w...
Summary: Caspofungin is an anti-fungal drug mainly metabolized by the liver. The pathophysiological status of children with severe infection will affect the metabolism of caspofungin in the body especially in the case of liver dysfunction. There is little metabolism of caspofungin through the kidney and continuous renal replacement therapy and renal function have little influence on the pharmacokinetics of...
Summary: Pneumocystis jirovecii pneumonia is a significant concern in peaple with HIV/AIDS, often severe and potentially fatal. While trimethoprim/sulfamethoxazole remains the primary treatment, safety concerns exist with alternative options. Research on Pneumocystis jirovecii's beta-D glucan composition has prompted investigations into echinocandins like caspofungin, showing promise in murine models and s...
Related Latest Advances
Brand Information
- Hypersensitivity
- Hepatic Effects
- Elevated Liver Enzymes During Concomitant Use With Cyclosporine
- Gastrointestinal disorders: pancreatitis
- Hepatobiliary disorders: hepatic necrosis
- Skin and subcutaneous tissue disorders: erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, and skin exfoliation
- Renal and urinary disorders: clinically significant renal dysfunction
- General disorders and administration site conditions: swelling and peripheral edema
- Laboratory abnormalities: gamma-glutamyltransferase increased
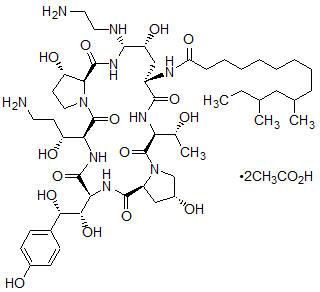
- Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med 1987 Oct 22;317(17): 1098 (letter).
(caspofungin acetate)
For Injection
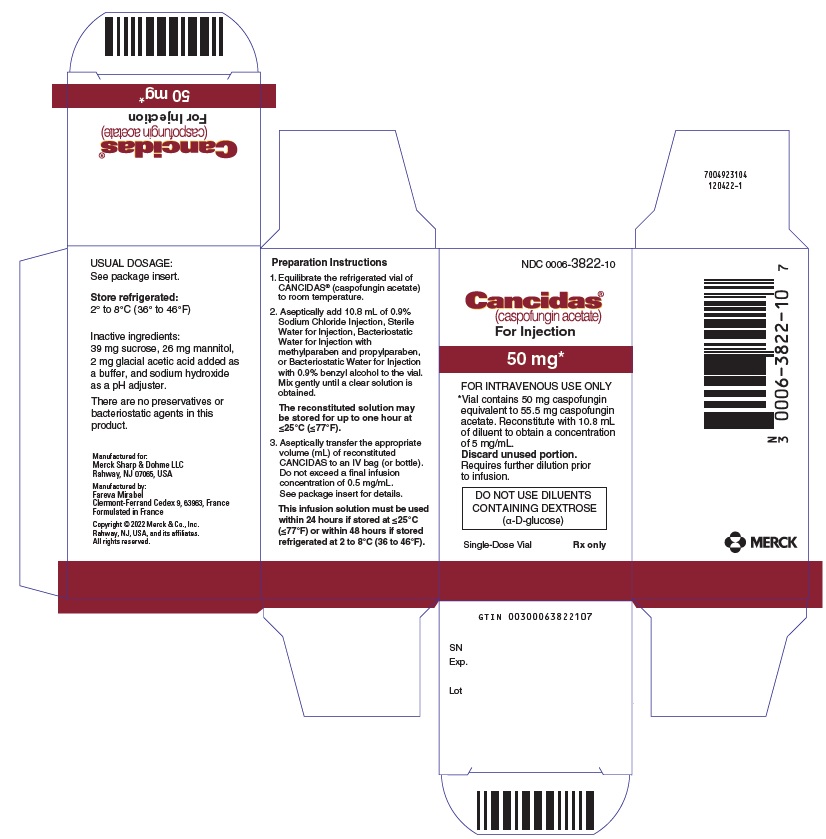
(caspofungin acetate)
For Injection