Mavenclad
What is Mavenclad (Cladribine)?
Approved To Treat
Related Clinical Trials
Summary: This phase I/II trial studies the side effects and how well cladribine, idarubicin, cytarabine, and quizartinib work in treating patients with acute myeloid leukemia or high-risk myelodysplastic syndrome that is newly diagnosed, has come back (relapsed), or does not respond to treatment (refractory). Drugs used in chemotherapy, such as cladribine, idarubicin, and cytarabine, work in different ways...
Summary: To learn about the safety and tolerability of study drug combinations in patients with relapsed/refractory, IDH1-mutated myeloid malignancies with a co-signaling mutation.
Summary: This is a multi-center prospective rater-masked (blinded) randomized controlled trial of 156 participants, comparing the treatment strategy of Autologous Hematopoietic Stem Cell Transplantation (AHSCT) to the treatment strategy of Best Available Therapy (BAT) for treatment-resistant relapsing multiple sclerosis (MS). Participants will be randomized at a 1 to 1 (1:1) ratio. All participants will be...
Related Latest Advances
Brand Information
- Malignancies
- Risk of Teratogenicity
- in patients with current malignancy
- in pregnant women and in women and men of reproductive potential who do not plan to use effective contraception during MAVENCLAD dosing and for 6 months after the last dose in each treatment course. May cause fetal harm
- in patients infected with the human immunodeficiency virus (HIV
- in patients with active chronic infections (e.g., hepatitis or tuberculosis)
- in patients with a history of hypersensitivity to cladribine
- in women intending to breastfeed on a MAVENCLAD treatment day and for 10 days after the last dose
- Malignancies
- Risk of Teratogenicity
- Lymphopenia
- Infections
- Hematologic Toxicity
- Graft-Versus-Host Disease With Blood Transfusion
- Liver Injury
- Hypersensitivity
- Cardiac Failure
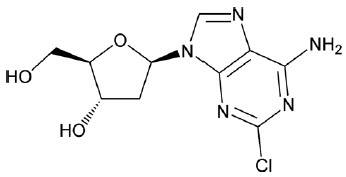
- "OSHA Hazardous Drugs". OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.