Brand Name
Invanz
Generic Name
Ertapenem
View Brand Information FDA approval date: November 21, 2001
Classification: Penem Antibacterial
Form: Injection
What is Invanz (Ertapenem)?
Ertapenem for injection is a penem antibacterial indicated in adult patients and pediatric patients for the treatment of the following moderate to severe infections caused by susceptible bacteria: Complicated intra-abdominal infections.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
INVANZ (ertapenem sodium)
1DOSAGE FORMS AND STRENGTHS
For Injection: Vials
INVANZ is a sterile lyophilized powder in a single-dose vial containing 1 g ertapenem equivalent to 1.046 g ertapenem sodium for intravenous infusion or for intramuscular injection after reconstitution.
2CONTRAINDICATIONS
- INVANZ is contraindicated in patients with known hypersensitivity to any component of this product or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams.
- Due to the use of lidocaine HCl as a diluent, INVANZ administered intramuscularly is contraindicated in patients with a known hypersensitivity to local anesthetics of the amide type.
3ADVERSE REACTIONS
The following are described in greater detail in the Warnings and Precautions section.
- Hypersensitivity Reactions
- Seizure Potential
- Interaction with Valproic Acid
- Clostridioides difficile-Associated Diarrhea (CDAD) [see
- Caution with Intramuscular Administration
- Development of Drug-Resistant Bacteria
- Laboratory Tests
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults Receiving INVANZ as a Treatment Regimen
Clinical trials enrolled 1954 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial
In patients treated for complicated intra-abdominal infections, death occurred in 4.7% (15/316) of patients receiving INVANZ and 2.6% (8/307) of patients receiving comparator drug. These deaths occurred in patients with significant co-morbidity and/or severe baseline infections. Deaths were considered unrelated to study drugs by investigators.
In clinical trials, seizure was reported during study therapy plus 14-day follow-up period in 0.5% of patients treated with INVANZ, 0.3% of patients treated with piperacillin/tazobactam and 0% of patients treated with ceftriaxone
Additional adverse experiences that were reported with INVANZ with an incidence >0.1% within each body system are listed below
Body as a Whole: abdominal distention, pain, chills, septicemia, septic shock, dehydration, gout, malaise, asthenia/fatigue, necrosis, candidiasis, weight loss, facial edema, injection site induration, injection site pain, extravasation, phlebitis/thrombophlebitis, flank pain, syncope
Cardiovascular System: heart failure, hematoma, chest pain, hypertension, tachycardia, cardiac arrest, bradycardia, arrhythmia, atrial fibrillation, heart murmur, ventricular tachycardia, asystole, subdural hemorrhage
Digestive System: acid regurgitation, oral candidiasis, dyspepsia, gastrointestinal hemorrhage, anorexia, flatulence, C. difficile-associated diarrhea, stomatitis, dysphagia, hemorrhoids, ileus, cholelithiasis, duodenitis, esophagitis, gastritis, jaundice, mouth ulcer, pancreatitis, pyloric stenosis
Musculoskeletal System: leg pain
Nervous System & Psychiatric: anxiety, nervousness, seizure [see , tremor, depression, hypesthesia, spasm, paresthesia, aggressive behavior, vertigo
Respiratory System: cough, pharyngitis, rales/rhonchi, respiratory distress, pleural effusion, hypoxemia, bronchoconstriction, pharyngeal discomfort, epistaxis, pleuritic pain, asthma, hemoptysis, hiccups, voice disturbance
Skin & Skin Appendage: erythema, sweating, dermatitis, desquamation, flushing, urticaria
Special Senses: taste perversion
Urogenital System: renal impairment, oliguria/anuria, vaginal pruritus, hematuria, urinary retention, bladder dysfunction, vaginal candidiasis, vulvovaginitis.
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the adverse experience profile was generally similar to that seen in previous clinical trials.
Prophylaxis of Surgical Site Infection following Elective Colorectal Surgery
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post-surgery, the overall adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials.
Additional adverse experiences that were reported in this prophylaxis trial with INVANZ, regardless of causality, with an incidence >0.5% within each body system are listed below:
Gastrointestinal Disorders: C. difficile infection or colitis, dry mouth, hematochezia
General Disorders and Administration Site Condition: crepitations
Infections and Infestations: cellulitis, abdominal abscess, fungal rash, pelvic abscess
Injury, Poisoning and Procedural Complications: incision site complication, incision site hemorrhage, intestinal stoma complication, anastomotic leak, seroma, wound dehiscence, wound secretion
Musculoskeletal and Connective Tissue Disorders: muscle spasms
Nervous System Disorders: cerebrovascular accident
Renal and Urinary Disorders: dysuria, pollakiuria
Respiratory, Thoracic and Mediastinal Disorders: crackles lung, lung infiltration, pulmonary congestion, pulmonary embolism, wheezing.
Pediatric Patients Receiving INVANZ as a Treatment Regimen
Clinical trials enrolled 384 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial
Additional adverse experiences that were reported with INVANZ with an incidence >0.5% within each body system are listed below:
Gastrointestinal Disorders: nausea
General Disorders and Administration Site Condition: hypothermia, chest pain, upper abdominal pain; infusion site pruritus, induration, phlebitis, swelling, and warmth
Infections and Infestations: candidiasis, oral candidiasis, viral pharyngitis, herpes simplex, ear infection, abdominal abscess
Metabolism and Nutrition Disorders: decreased appetite
Musculoskeletal and Connective Tissue Disorders: arthralgia
Nervous System Disorders: dizziness, somnolence
Psychiatric Disorders: insomnia
Reproductive System and Breast Disorders: genital rash
Respiratory, Thoracic and Mediastinal Disorders: wheezing, nasopharyngitis, pleural effusion, rhinitis, rhinorrhea
Skin and Subcutaneous Tissue Disorders: dermatitis, pruritus, rash erythematous, skin lesion
Vascular Disorders: phlebitis.
3.2Post-Marketing Experience
The following additional adverse reactions have been identified during the post-approval use of INVANZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: teeth staining
Immune System Disorders: anaphylaxis including anaphylactoid reactions
Musculoskeletal and Connective Tissue Disorders: muscular weakness
Nervous System Disorders: coordination abnormal, depressed level of consciousness, dyskinesia, gait disturbance, myoclonus, tremor, encephalopathy (recovery was prolonged in patients with renal impairment)
Psychiatric Disorders: altered mental status (including aggression, delirium), hallucinations
Skin and Subcutaneous Tissue Disorders: Acute Generalized Exanthematous Pustulosis (AGEP), Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS syndrome), hypersensitivity vasculitis
3.3Adverse Laboratory Changes in Clinical Trials
Adults Receiving INVANZ as Treatment Regimen
Laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ in clinical trials are presented in
Additional laboratory adverse experiences that were reported during therapy in >0.1% of patients treated with INVANZ in clinical trials include: increases in serum creatinine, serum glucose, BUN, total, direct and indirect serum bilirubin, serum sodium and potassium, PT and PTT; decreases in serum potassium, serum albumin, WBC, platelet count, and segmented neutrophils.
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the laboratory adverse experience profile was generally similar to that seen in previous clinical trials.
Prophylaxis of Surgical Site Infection following Elective Colorectal Surgery
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post-surgery, the overall laboratory adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials.
Pediatric Patients Receiving INVANZ as a Treatment Regimen
Laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ in clinical trials are presented in
Additional laboratory adverse experiences that were reported during therapy in >0.5% of patients treated with INVANZ in clinical trials include: alkaline phosphatase increased, eosinophil count increased, platelet count increased, white blood cell count decreased and urine protein present.
4OVERDOSAGE
No specific information is available on the treatment of overdosage with INVANZ. Intentional overdosing of INVANZ is unlikely. Intravenous administration of INVANZ at a dose of 2 g over 30 min or 3 g over 1-2h in healthy adult volunteers resulted in an increased incidence of nausea. In clinical trials in adults, inadvertent administration of three 1 g doses of INVANZ in a 24 hour period resulted in diarrhea and transient dizziness in one patient. In pediatric clinical trials, a single intravenous dose of 40 mg/kg up to a maximum of 2 g did not result in toxicity.
In the event of an overdose, INVANZ should be discontinued and general supportive treatment given until renal elimination takes place.
INVANZ can be removed by hemodialysis; the plasma clearance of the total fraction of ertapenem was increased 30% in subjects with end-stage renal disease when hemodialysis (4 hour session) was performed immediately following administration. However, no information is available on the use of hemodialysis to treat overdosage.
5DESCRIPTION
INVANZ (Ertapenem for Injection) is a sterile, synthetic, parenteral, 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics.
Chemically, INVANZ is described as [4
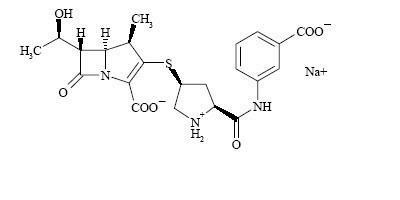
Ertapenem sodium is a white to off-white hygroscopic, weakly crystalline powder. It is soluble in water and 0.9% sodium chloride solution, practically insoluble in ethanol, and insoluble in isopropyl acetate and tetrahydrofuran.
INVANZ is supplied as sterile lyophilized powder for intravenous infusion after reconstitution with appropriate diluent
Each vial of INVANZ contains the following inactive ingredients: 175 mg sodium bicarbonate and sodium hydroxide to adjust pH to 7.5.
6PRINCIPAL DISPLAY PANEL - 10 Single-dose vials Label
NDC 0006-3843-71
10 Single-dose vials
FOR INSTRUCTIONS ON RECONSTITUTION
Each vial contains 1 gram ertapenem,
INVANZ®
(ertapenem sodium) IV/IM
(ertapenem sodium) IV/IM
FOR INTRAVENOUS OR
Rx only
Inactive ingredients: 175 mg sodium
Solutions range from colorless to pale
Do not store lyophilized powder above
MERCK
Manuf. for: Merck Sharp & Dohme LLC
By: Fareva Mirabel
