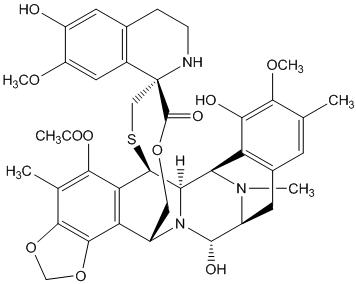
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to YONDELIS in 755 patients with soft tissue sarcoma including 197 (26%) patients exposed to YONDELIS for greater than or equal to 6 months and 57 (8%) patients exposed to YONDELIS for greater than or equal to 1 year. The safety of YONDELIS was evaluated in six open-label, single-arm trials, in which 377 patients received YONDELIS and one open-label, randomized, active-controlled clinical trial in which 378 patients received YONDELIS (Trial ET743-SAR-3007). All patients received YONDELIS at the recommended dosing regimen of 1.5 mg/m
Tables 3 and 4 present selected adverse reactions and laboratory abnormalities, respectively, observed in Trial ET743-SAR-3007, an open-label, randomized (2:1), active-controlled trial in which 550 patients with previously treated leiomyosarcoma or liposarcoma (dedifferentiated, myxoid round cell, or pleomorphic) received YONDELIS 1.5 mg/m
In Trial ET743-SAR-3007, patients had been previously treated with an anthracycline- and ifosfamide-containing regimen or with an anthracycline-containing regimen and one additional cytotoxic chemotherapy regimen. The trial excluded patients with known central nervous system metastasis, elevated serum bilirubin or significant chronic liver disease, such as cirrhosis or active hepatitis, and history of myocardial infarction within 6 months, history of New York Heart Association Class II to IV heart failure, or abnormal left ventricular ejection fraction at baseline. The median age of patients in Trial ET743-SAR-3007 was 57 years (range: 17 to 81 years), with 69% female, 77% White, 12% Black or African American, 4% Asian, and <1% American Indian or Alaska Native. The median duration of exposure to trabectedin was 13 weeks (range: 1 to 127 weeks) with 30% of patients exposed to YONDELIS for greater than 6 months and 7% of patients exposed to YONDELIS for greater than 1 year.
In Trial ET743-SAR-3007, adverse reactions resulting in permanent discontinuation of YONDELIS occurred in 26% (98/378) of patients; the most common were increased liver tests (defined as ALT, AST, alkaline phosphatase, bilirubin) (5.6%), thrombocytopenia (3.4%), fatigue (1.6%), increased creatine phosphokinase (1.1%), and decreased ejection fraction (1.1%). Adverse reactions that led to dose reductions occurred in 42% (158/378) of patients treated with YONDELIS; the most common were increased liver tests (24%), neutropenia (including febrile neutropenia) (8%), thrombocytopenia (4.2%), fatigue (3.7%), increased creatine phosphokinase (2.4%), nausea (1.1%), and vomiting (1.1%). Adverse reactions led to dose interruptions in 52% (198/378) of patients treated with YONDELIS; the most common were neutropenia (31%), thrombocytopenia (15%), increased liver tests (6%), fatigue (2.9%), anemia (2.6%), increased creatinine (1.1%), and nausea (1.1%).
The most common adverse reactions (≥20%) were nausea, fatigue, vomiting, constipation, decreased appetite, diarrhea, peripheral edema, dyspnea, and headache. The most common laboratory abnormalities (≥20%) were increases in AST or ALT, increased alkaline phosphatase, hypoalbuminemia, increased creatinine, increased creatine phosphokinase, anemia, neutropenia, and thrombocytopenia.
Other clinically important adverse reactions observed in <10% of patients (N=755) with soft tissue sarcoma receiving YONDELIS were:
Nervous system disorders: peripheral neuropathy, paresthesia, hypoesthesia.
Respiratory, thoracic, and mediastinal disorders: pulmonary embolism.
General disorders and administration site conditions: mucosal inflammation.