Generic Name
Dexmethylphenidate
Brand Names
Focalin, Azstarys
FDA approval date: November 30, 2001
Classification: Central Nervous System Stimulant
Form: Tablet, Capsule
What is Focalin (Dexmethylphenidate)?
Dexmethylphenidate hydrochloride tablets is indicated for the treatment of Attention Deficit Hyperactivity Disorder . Dexmethylphenidate hydrochloride tablets is a central nervous system stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Focalin (dexmethylphenidate hydrochloride)
WARNING: ABUSE, MISUSE, AND ADDICTION
F
Before prescribing Focalin XR, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout Focalin XR treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction
1INDICATIONS AND USAGE
Focalin XR is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD)
Limitations of Use
The use of Focalin XR is not recommended in pediatric patients younger than 6 years of age because they had higher plasma exposure and a higher incidence of adverse reactions (e.g., weight loss) than patients 6 years and older at the same dosage
2DOSAGE FORMS AND STRENGTHS
- 5 mg extended-release capsules – light blue opaque cap and body (imprinted with “NVR” on cap and “D5” on the body)
- 10 mg extended-release capsules – light caramel opaque cap and body (imprinted with “NVR” on cap and “D10” on the body)
- 15 mg extended-release capsules – green opaque cap and body (imprinted with “NVR” on cap and “D15” on the body)
- 20 mg extended-release capsules – white opaque cap and body (imprinted with “NVR” on cap and “D20” on the body)
- 25 mg extended-release capsules – light blue opaque cap and white opaque body (imprinted with “NVR” on cap and “D25” on the body)
- 30 mg extended-release capsules – light caramel opaque cap and white opaque body (imprinted with “NVR” on cap and “D30” on the body)
- 35 mg extended-release capsules – light blue opaque cap and light caramel opaque body (imprinted with “NVR” on cap and “D35” on the body)
- 40 mg extended-release capsules – green opaque cap and white opaque body (imprinted with “NVR” on cap and “D40” on the body)
3CONTRAINDICATIONS
- Hypersensitivity to methylphenidate or other components of Focalin XR. Hypersensitivity reactions, such as angioedema and anaphylactic reactions have been reported in patients treated with methylphenidate
- Concomitant treatment with monoamine oxidase inhibitors (MAOIs) or within 14 days following discontinuation of treatment with an MAOI, because of the risk of hypertensive crises
4ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Abuse, Misuse, and Addiction
- Known hypersensitivity to methylphenidate or other ingredients of Focalin XR
- Hypertensive Crisis with Concomitant Use of Monoamine Oxidase Inhibitors
- Risks to Patients with Serious Cardiac Disease
- Increased Blood Pressure and Heart Rate
- Psychiatric Adverse Reactions
- Priapism
- Peripheral Vasculopathy, Including Raynaud’s Phenomenon
- Long-Term Suppression of Growth in Pediatric Patients
- Acute Angle Closure Glaucoma
- Increased Intraocular Pressure and Glaucoma
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Studies with Focalin XR in Pediatric Patients with ADHD
The safety data in this section is based on data from a 7-week controlled clinical study of Focalin XR in 100 (103 randomized) pediatric patients with ADHD ages 6 to 17 years (ages 6 to 12, n = 86; ages 13 to 17, n = 17).
This study was a randomized, double-blind, placebo-controlled, parallel-group study to evaluate the time of onset, duration of efficacy, tolerability, safety of Focalin XR 5 mg to 30 mg/day who met The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for ADHD
Most Common Adverse Reactions (incidence of greater than or equal to 5% and at least twice placebo): dyspepsia, decreased appetite, headache, and anxiety.
Adverse Reactions Leading to Discontinuation: 50 of 684 (7.3%) pediatric patients treated with Focalin (dexmethylphenidate) immediate-release tablets experienced an adverse reaction that resulted in discontinuation. The most common reasons for discontinuation were twitching (described as motor or vocal tics), anorexia, insomnia, and tachycardia (approximately 1% each).
Table 1 enumerates adverse reactions for the placebo-controlled, parallel-group study in children and adolescents with ADHD at flexible Focalin XR doses of 5–30 mg/day. The table includes only those events that occurred in 5% or more of patients treated with Focalin XR and for which the incidence in patients treated with Focalin XR was at least twice the incidence in placebo-treated patients.
Table 2 below enumerates the incidence of dose-related adverse reactions that occurred during a fixed-dose, double-blind, placebo-controlled trial in pediatric patients with ADHD taking Focalin XR up to 30 mg daily versus placebo. The table includes only those reactions that occurred in patients treated with Focalin XR for which the incidence was at least 5% and greater than the incidence among placebo-treated patients.
Adverse Reactions in Studies with Focalin XR in Adult Patients with ADHD
The safety data in this section is based on data from a 5-week controlled clinical study of Focalin XR in 218 adult patients (221 randomized) with ADHD ages 18 to 60 years. In this study, 101 adult patients were treated for at least 6 months.
This study was a randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy, safety, and tolerability of Focalin XR 20 mg, 30 mg, or 40 mg daily who met DSM-IV criteria for ADHD
Most Common Adverse Reactions (incidence of greater than or equal to 5% and at least twice placebo): dry mouth, dyspepsia, headache, anxiety, and pharyngolaryngeal pain.
Adverse Reactions Leading to Discontinuation: During the double-blind phase of the study, 10.7% of the Focalin XR-treated patients and 7.5% of the placebo-treated patients discontinued due to adverse reactions. Three patients (1.8%) in the Focalin XR discontinued due to insomnia and jittery respectively; and two patients (1.2%) in the Focalin XR discontinued due to anorexia and anxiety, respectively.
Table 3 enumerates adverse reactions for the placebo-controlled, parallel-group study in adults with ADHD at fixed Focalin XR doses of 20, 30, or 40 mg/day. The table includes only those events that occurred in 5% or more of patients in a Focalin XR dose group and for which the incidences in patients treated with Focalin XR appeared to increase with dose.
Two other adverse reactions occurring in clinical trials with Focalin XR at a frequency greater than placebo, but which were not dose related were: feeling jittery (12% and 2%, respectively) and dizziness (6% and 2%, respectively).
Table 4 summarizes changes in vital signs and weight that were recorded in the adult study (N = 218) of Focalin XR in the treatment of ADHD.
4.2Postmarketing Experience
The following additional adverse reactions have been identified during postapproval use of dexmethylphenidate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal: rhabdomyolysis
Immune System Disorders: hypersensitivity reactions, including angioedema and anaphylaxis
Adverse Reactions Reported With All Ritalin and Focalin Formulations
The following adverse reactions associated with the use of all Ritalin and Focalin formulations were identified in clinical trials, spontaneous reports, and literature. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Infections and Infestations: nasopharyngitis
Blood and the Lymphatic System Disorders: leukopenia, thrombocytopenia, anemia
Immune System Disorders: hypersensitivity reactions, including angioedema and anaphylaxis
Metabolism and Nutrition Disorders: decreased appetite, reduced weight gain, and suppression of growth during prolonged use in pediatric patients
Psychiatric Disorders: insomnia, anxiety, restlessness, agitation, psychosis (sometimes with visual and tactile hallucinations), depressed mood, depression
Nervous System Disorders: headache, dizziness, tremor, dyskinesia, including choreoathetoid movements, drowsiness, convulsions, cerebrovascular disorders (including vasculitis, cerebral hemorrhages and cerebrovascular accidents), serotonin syndrome in combination with serotonergic drugs
Eye Disorders: blurred vision, difficulties in visual accommodation
Cardiac Disorders: tachycardia, palpitations, increased blood pressure, arrhythmias, angina pectoris
Respiratory, Thoracic, and Mediastinal Disorders: cough
Gastrointestinal Disorders: dry mouth, nausea, vomiting, abdominal pain, dyspepsia
Hepatobiliary Disorders: abnormal liver function, ranging from transaminase elevation to severe hepatic injury
Skin and Subcutaneous Tissue Disorders: hyperhidrosis, pruritus, urticaria, exfoliative dermatitis, scalp hair loss, erythema multiforme rash, thrombocytopenic purpura
Musculoskeletal and Connective Tissue Disorders: arthralgia, muscle cramps, rhabdomyolysis, trismus
Investigations: weight loss (adult ADHD patients)
Vascular Disorders: peripheral coldness, Raynaud's phenomenon
Additional Adverse Reactions Reported with Other Methylphenidate Products
The list below shows adverse reactions not listed with Ritalin and Focalin formulations that have been reported with other methylphenidate products based on clinical trials data and post-marketing spontaneous reports.
Blood and Lymphatic Disorders: pancytopenia
Immune System Disorders: hypersensitivity reactions, such as auricular swelling, bullous conditions, eruptions, exanthemas
Psychiatric Disorders: affect lability, mania, disorientation, libido changes
Nervous System Disorders: migraine, motor and verbal tics
Eye Disorders: diplopia, increased intraocular pressure, mydriasis
Cardiac Disorders: sudden cardiac death, myocardial infarction, bradycardia, extrasystole, supraventricular tachycardia, ventricular extrasystole
Respiratory, Thoracic, and Mediastinal Disorders: pharyngolaryngeal pain, dyspnea
Gastrointestinal Disorders: diarrhea, constipation
Skin and Subcutaneous Tissue Disorders: angioneurotic edema, erythema, fixed drug eruption
Musculoskeletal, Connective Tissue, and Bone Disorders: myalgia, muscle twitching
Renal and Urinary Disorders: hematuria
Reproductive System and Breast Disorders: gynecomastia
General Disorders: fatigue, hyperpyrexia
Urogenital Disorders: priapism
5OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Consider the possibility of multiple drug ingestion. The pharmacokinetic profile of Focalin XR should be considered when treating patients with overdose. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
6DESCRIPTION
Focalin XR contains dexmethylphenidate hydrochloride, a CNS stimulant. Dexmethylphenidate hydrochloride is the
Chemically, dexmethylphenidate hydrochloride is methyl α-phenyl-2-piperidineacetate hydrochloride, (R,R’)-(+)-. Its molecular formula is C
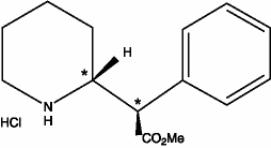
Note* = asymmetric carbon center
Dexmethylphenidate hydrochloride is a white to off-white powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77 g/mol.
Inactive ingredients: ammonio methacrylate copolymer, gelatin, methacrylic acid copolymer, polyethylene glycol, sugar spheres, talc, titanium dioxide, and triethyl citrate.
Each strength capsule also contains colorant ingredients in the capsule shell as follows:
- 5 mg: E132 FD&C Blue No. 2
- 10 mg: FDA/E172 iron oxide yellow
- 15 mg: FD&C Blue No. 2, FDA/E172 iron oxide yellow
- 20 mg: contains no colorants
- 25 mg: E132 FD&C Blue No. 2
- 30 mg: FDA/E172 iron oxide yellow
- 35 mg: E132 FD&C Blue No. 2, FDA/E172 iron oxide yellow
- 40 mg: E132 FD&C Blue No. 2, FDA/E172 iron oxide yellow
7HOW SUPPLIED/STORAGE AND HANDLING
Focalin XR (dexmethylphenidate hydrochloride) extended-release capsules are available as follows:
- 5 mg capsules (NDC 0078-0430-05) light-blue, (imprinted “NVR D5”) supplied in bottles of 100
- 10 mg capsules (NDC 0078-0431-05) light caramel (imprinted “NVR D10”) supplied in bottles of 100
- 15 mg capsules (NDC 0078-0493-05) green (imprinted “NVR D15”) supplied in bottles of 100
- 20 mg capsules (NDC 0078-0432-05) white (imprinted “NVR D20”) supplied in bottles of 100
- 25 mg capsules (NDC 0078-0608-05) light-blue and white (imprinted “NVR D25”) supplied in bottles of 100
- 30 mg capsules (NDC 0078-0433-05) light caramel and white (imprinted “NVR D30”) supplied in bottles of 100
- 35 mg capsules (NDC 0078-0609-05) light-blue and light caramel (imprinted “NVR D35”) supplied in bottles of 100
- 40 mg capsules (NDC 0078-0434-05) green and white (imprinted “NVR D40”) supplied in bottles of 100
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Dispense in tight container (USP).
8PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of Focalin XR, which can lead to overdose and death, and proper disposal of any unused drug
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with Focalin XR use. Instruct patients to contact a healthcare provider immediately if they develop symptoms, such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease
Increased Blood Pressure and Heart Rate
Instruct patients that Focalin XR can cause elevations of their blood pressure and pulse rate
Psychiatric Adverse Reactions
Advise patients that Focalin XR, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct them to seek immediate medical attention in the event of priapism
Circulation Problems in Fingers and Toes (Peripheral Vasculopathy, Including Raynaud’s Phenomenon)
Instruct patients beginning treatment with Focalin XR about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking Focalin XR. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients
Long-Term Suppression of Growth in Pediatric Patients
Advise patients that Focalin XR may cause slowing of growth and weight loss
Increased Intraocular Pressure (IOP) and Glaucoma
Advise patients that IOP and glaucoma may occur during treatment with Focalin XR
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s Syndrome may occur during treatment with Focalin XR. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette’s syndrome occurs
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to ADHD medications, including Focalin XR, during pregnancy
Distributed by:
9PRINCIPAL DISPLAY PANEL
NDC 0078-0430-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
5 mg
100 capsules
Dispense with Medication
NOVARTIS

10PRINCIPAL DISPLAY PANEL
NDC 0078-0431-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
10 mg
100 capsules
Dispense with Medication
NOVARTIS

11PRINCIPAL DISPLAY PANEL
NDC 0078-0493-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
15 mg
100 capsules
Dispense with Medication
NOVARTIS

12PRINCIPAL DISPLAY PANEL
NDC 0078-0432-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
20 mg
100 capsules
Dispense with Medication
NOVARTIS

13PRINCIPAL DISPLAY PANEL
NDC 0078-0608-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
25 mg
100 capsules
Dispense in tight container (USP).
Dispense with Medication Guide
NOVARTIS

14PRINCIPAL DISPLAY PANEL
NDC 0078-0433-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
30 mg
100 capsules
Dispense in tight container (USP).
Dispense with
NOVARTIS

15PRINCIPAL DISPLAY PANEL
NDC 0078-0609-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
35 mg
100 capsules
Dispense in tight container (USP).
Dispense with
NOVARTIS

16PRINCIPAL DISPLAY PANEL
NDC 0078-0434-05 Rx only
Focalin XR
(dexmethylphenidate HCl)
extended-release capsules
40 mg
100 capsules
Dispense in tight container (USP).
Dispense with
NOVARTIS
