Intuniv
What is Intuniv (Guanfacine)?
For a parent or caregiver of a child with Attention-Deficit Hyperactivity Disorder (ADHD), navigating daily life can be a challenge. Helping your child manage symptoms like inattention, impulsivity, and hyperactivity requires a toolkit of strategies, often including therapy, school support, and sometimes, medication. While stimulant medications are a common and effective choice, they aren’t the right fit for every child. This is where non-stimulant options like Intuniv (guanfacine) play a vital role.
Intuniv is a prescription medication approved to treat ADHD in children and adolescents, typically between the ages of 6 and 17. It belongs to a class of drugs known as alpha-2A adrenergic receptor agonists. Unlike stimulants, which work by increasing certain brain chemicals, Intuniv offers a different approach to improving focus and reducing impulsivity. It can be used on its own or in combination with a stimulant medication, providing a flexible and personalized option for managing ADHD symptoms.
What does Intuniv do?
Intuniv is primarily prescribed for the treatment of ADHD. Its goal is to help reduce the core symptoms of the condition, which can lead to significant improvements in a child’s daily functioning. Parents and teachers often notice positive changes in several key areas.
A child taking Intuniv may experience:
- Improved ability to pay attention and stay on task
- Reduced impulsivity and hyperactivity
- Better emotional regulation and less frustration
- Enhanced ability to follow directions and complete schoolwork
Intuniv’s full benefits appear gradually over weeks as the dose increases, unlike immediate stimulants. Clinical studies have shown that Intuniv is effective in significantly reducing ADHD symptoms compared to a placebo, helping children better manage their behavior at home, in school, and during social activities (Takeda Pharmaceuticals America, Inc., 2021).
How does Intuniv work?
To understand how Intuniv works, it’s helpful to think about the brain’s “command center,” the prefrontal cortex. This area is responsible for executive functions, which include paying attention, planning, organizing, and controlling impulses. In individuals with ADHD, the communication network in this part of the brain may not be working as efficiently as it should.
Intuniv is thought to work by strengthening the signals between nerve cells in the prefrontal cortex. It does this by targeting and stimulating specific protein receptors on these cells, known as alpha-2A adrenergic receptors. By activating these receptors, Intuniv helps to improve the brain’s communication pathways, making it easier for a child to regulate their attention and behavior.
Unlike stimulants that boost overall dopamine and norepinephrine, Intuniv, a non-stimulant, acts specifically in the prefrontal cortex. This targeted approach offers an alternative for children who react poorly to or experience side effects from stimulants.
Intuniv side effects
Like all medications, Intuniv can cause side effects. Most are mild to moderate and often decrease over time as the body adjusts. It is important to discuss any concerns with your child’s doctor.
The most common side effects of Intuniv include:
- Drowsiness or sleepiness (somnolence), which is most common when starting the medication or increasing the dose
- Fatigue or feeling tired
- Headache
- Abdominal pain
- Dizziness
- Low blood pressure (hypotension)
- A slower than normal heart rate (bradycardia)
Because Intuniv can lower blood pressure and heart rate, it’s important for your child to avoid becoming dehydrated or overheated. Your doctor will monitor these vital signs.
Serious side effects are rare but possible. You should contact your doctor if your child faints, feels very dizzy, or seems unusually tired. It is crucial not to stop Intuniv abruptly, as this can cause a rapid increase in blood pressure. The medication must be tapered down slowly under a doctor’s guidance. Seek immediate medical help for any signs of a severe allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat (National Institutes of Health, 2018).
Intuniv dosage
Intuniv is an extended-release tablet that is taken once a day, either in the morning or the evening. Take the tablet whole with liquid around the same time daily. Do not crush, chew, or break it, as this damages the extended-release coating and causes rapid medication release.
A doctor will regularly monitor your child’s blood pressure and heart rate before and throughout treatment, especially after dose changes, to ensure safety and effectiveness. These checks are essential for managing potential side effects like dizziness or low blood pressure. Your doctor will start with a low dose and increase it gradually over several weeks to find the optimal dose that provides the most benefit with the fewest side effects.
Does Intuniv have a generic version?
Yes, Intuniv is available in a generic form. The generic name is guanfacine extended release. The U.S. Food and Drug Administration (FDA) requires that generic medications be bioequivalent to their brand-name counterparts, which means they provide the same clinical benefit and are just as safe and effective.
The availability of a generic version can make the treatment more affordable and accessible for many families. Whether your child is prescribed brand-name Intuniv or its generic equivalent, you can be confident they are receiving the same active ingredient and therapeutic effect.
Conclusion
Intuniv (guanfacine) offers a valuable non-stimulant treatment option for children and adolescents with ADHD. By working differently from stimulants, it provides an effective way to improve focus, reduce impulsivity, and enhance overall daily functioning. For many families, it can be a primary therapy or a helpful addition to a comprehensive ADHD management plan.
While it has potential side effects, most are manageable and can be minimized through careful, gradual dosing and regular monitoring by a healthcare provider. Every child’s journey with ADHD is unique, and finding the right support is key. By working closely with your child’s doctor, you can determine if Intuniv is the right choice to help them thrive.
References
- National Institutes of Health. (2018). Guanfacine. MedlinePlus. https://medlineplus.gov/druginfo/meds/a601058.html
- Takeda Pharmaceuticals America, Inc. (2021). INTUNIV® (guanfacine) Prescribing Information. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022037s028lbl.pdf
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Hypotension, bradycardia, and syncope
- Sedation and somnolence
- Cardiac conduction abnormalities
- Rebound Hypertension [see
- Five short-term, placebo-controlled monotherapy trials (Studies 1, 2, 4, 5, and 6).
- One short-term, placebo-controlled adjunctive trial with psychostimulants (Study 3).
- One long-term, placebo-controlled monotherapy maintenance trial (Study 7).
- have heart problems or a low heart rate
- have fainted
- have low or high blood pressure
- have liver or kidney problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if INTUNIV will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if INTUNIV passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking INTUNIV.
- ketoconazole
- medicines that can affect enzyme metabolism
- high blood pressure medicine
- sedatives
- benzodiazepines
- barbiturates
- antipsychotics
- Take INTUNIV exactly as your doctor tells you.
- Your doctor may change your dose. Do not change your dose of INTUNIV without talking to your doctor.
- Do not stop taking INTUNIV without talking to your doctor.
- Try not to miss your dose of INTUNIV. If you miss a dose of INTUNIV, take the next dose at your regular time. If you miss 2 or more doses, talk to your doctor, as you may need to restart INTUNIV with a lower dose.
- Do not take a double dose to make up for a missed dose.
- INTUNIV should be taken 1 time a day in the morning or in the evening, either alone or in combination with an ADHD stimulant medicine that your doctor may prescribe. Your doctor will tell you when to take INTUNIV and when to take your ADHD stimulant medication.
- INTUNIV should be swallowed whole with a small amount of water, milk, or other liquid.
- Do not crush, chew, or break INTUNIV. Tell your doctor if you cannot swallow INTUNIV whole.
- Do not take INTUNIV with a high-fat meal.
- Your doctor will check your blood pressure and heart rate while you take INTUNIV.
- If you take too much INTUNIV, call your local Poison Control Center at 1-800-222-1222 or go to the nearest emergency room right away.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how INTUNIV affects you. INTUNIV can slow your thinking and motor skills.
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking INTUNIV until you talk with your doctor. INTUNIV taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not become dehydrated or overheated. This may increase your chance of having low blood pressure or fainting while taking INTUNIV.
- Do not suddenly stop INTUNIV. Tell your healthcare provider if you have been vomiting and cannot take INTUNIV, you may be at risk for rebound hypertension.
- low blood pressure
- low heart rate
- fainting
- sleepiness
- increased blood pressure and heart rate after suddenly stopping INTUNIV (rebound hypertension). Suddenly stopping INTUNIV can cause increased blood pressure and heart rate and other withdrawal symptoms such as headache, confusion, nervousness, agitation, and tremors. If these symptoms continue to get worse and are left untreated, it could lead to a very serious condition including very high blood pressure, feeling very sleepy or tired, severe headache, vomiting, vision problems, seizures.
- sleepiness
- tiredness
- trouble sleeping
- low blood pressure
- nausea
- stomach pain
- dizziness
- dry mouth
- irritability
- vomiting
- slow heart rate
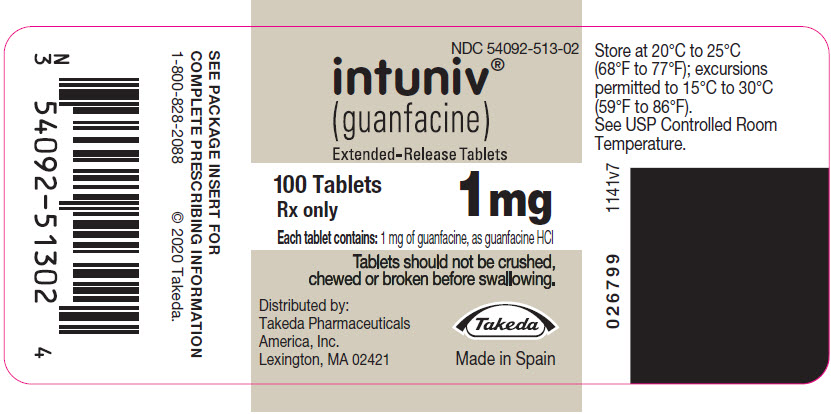
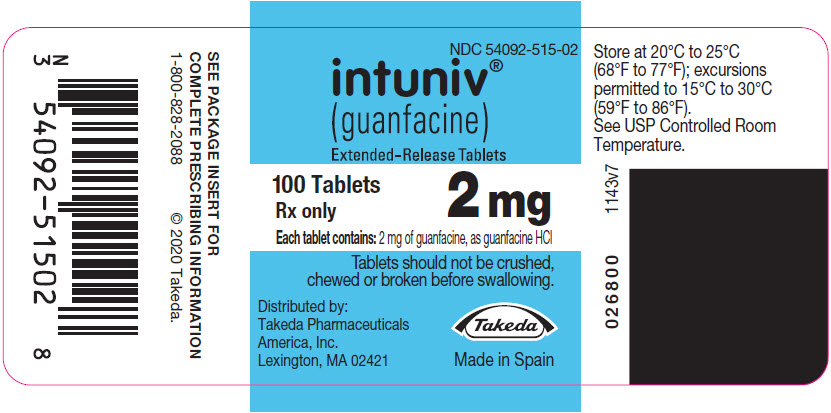
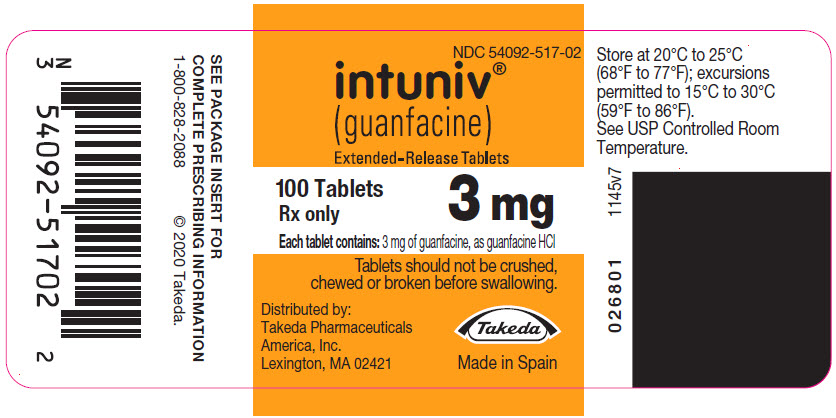
- Store INTUNIV between 68°F to 77°F (20°C to 25°C)
(guanfacine)
Extended-Release Tablets
(guanfacine)
Extended-Release Tablets
(guanfacine)
Extended-Release Tablets
(guanfacine)
Extended-Release Tablets

