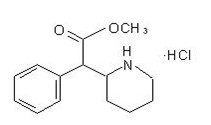
methYLphenidate
What is Relexxii (methYLphenidate)?
Approved To Treat
Related Clinical Trials
Summary: This study seeks to provide insight on psilocybin's effects on mechanisms of chronic pain among patients with co-morbid chronic low back pain and depression (CLBP+D). Participants will receive either a single high-dose of psilocybin (25mg absolute dose) or methylphenidate (40mg absolute dose). Participants will be asked to complete assessments of pain, depressive symptoms, and more general questio...
Summary: The aim of this study was to investigate the effect of treatment of ADHD with methylphenidate on neuroinflammation by examining the levels of Interleukin-6 (IL-6), S100B, Claudin-5 in serum samples of patients who were diagnosed with attention deficit hyperactivity disorder (ADHD) and started or planned to start methylphenidate for treatment as per routine, at month 0 before the initiation of meth...
Objectives: The objective is to determine the acute and chronic impact of the orexin antagonist, suvorexant, on neurobiological and behavioral factors linked with substance use disorders. Whether such effects are mediated by baseline characteristics will be tested. Given suvorexant is an FDA approved treatment for insomnia, sleep will be evaluated as well in the nicotine dependent arm. Endpoints: In nicotine-...
Related Latest Advances
Brand Information
- 18 mg: yellow with “TL706” imprinted in black ink
- 27 mg: gray with “TL707” imprinted in black ink
- 36 mg: white with “TL708” imprinted in black ink
- 45 mg: pink with “TL711” imprinted in black ink
- 54 mg: pink with “TL709” imprinted in black ink
- 63 mg: orange with “TL700” imprinted in black ink and
- 72 mg: blue with “TL710” imprinted in black ink.
- with a known hypersensitivity to methylphenidate or other components of RELEXXII. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other methylphenidate products
- receiving concomitant treatment with monoamine oxidase inhibitors (MAOIs), and also within 14 days following discontinuation of treatment with a MAOI, because of the risk of hypertensive crisis
- Abuse, Misuse, and Addiction
- Known hypersensitivity to methylphenidate or other ingredients
- Hypertensive crisis when used concomitantly with monoamine oxidase inhibitors
- Risks to Patients with Serious Cardiac Disease
- Increased Blood Pressure and Heart Rate
- Psychiatric Adverse Reactions
- Priapism
- Peripheral Vasculopathy, including Raynaud’s Phenomenon
- Long-Term Suppression of Growth in Pediatric Patients
- Potential for Gastrointestinal Obstruction
- Acute Angle Closure Glaucoma
- Increased Intraocular Pressure and Glaucoma
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
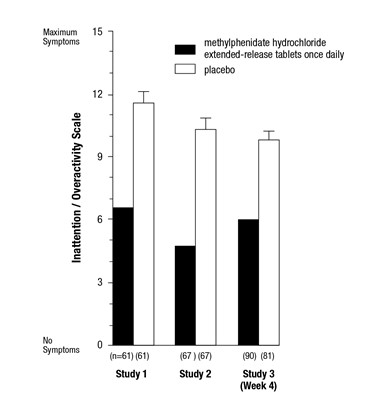
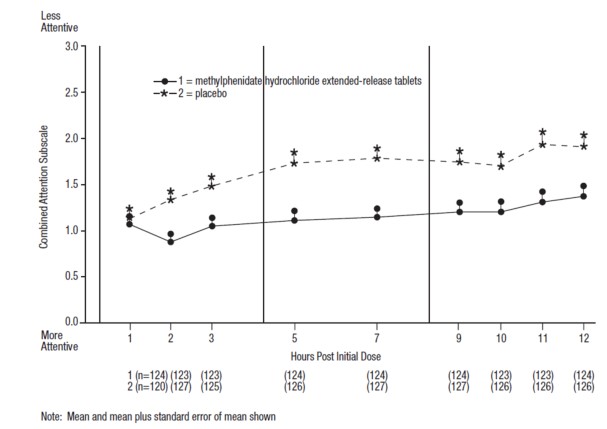
- Pediatric patients 6 to 17 years: abdominal pain upper (see Table 4).
- Adults: decreased appetite, headache, dry mouth, nausea, insomnia, anxiety, dizziness, weight decreased, irritability, and hyperhidrosis (see Table 5).
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
- 18 mg yellow tablets with "TL706" imprinted in black ink
- 27 mg gray tablets with "TL707" imprinted in black ink
- 36 mg white tablets with "TL708" imprinted in black ink
- 45 mg pink tablets with "TL711" imprinted in black ink
- 54 mg pink tablets with "TL709" imprinted in black ink
- 63 mg orange tablets with "TL700" imprinted in black ink
- 72 mg blue tablets with "TL710" imprinted in black ink
- Instruct patients beginning treatment with RELEXXII about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their healthcare provider any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking RELEXXII.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.













