Generic Name
Finasteride
Brand Names
Propecia, Proscar
FDA approval date: December 15, 2006
Classification: 5-alpha Reductase Inhibitor
Form: Tablet
What is Propecia (Finasteride)?
PROSCAR, is a 5α-reductase inhibitor, indicated for the treatment of symptomatic benign prostatic hyperplasia in men with an enlarged prostate to.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
PROPECIA (finasteride)
1INDICATIONS AND USAGE
PROPECIA
Efficacy in bitemporal recession has not been established.
PROPECIA is not indicated for use in women.
2DOSAGE AND ADMINISTRATION
PROPECIA may be administered with or without meals.
The recommended dose of PROPECIA is one tablet (1 mg) taken once daily.
In general, daily use for three months or more is necessary before benefit is observed. Continued use is recommended to sustain benefit, which should be re-evaluated periodically. Withdrawal of treatment leads to reversal of effect within 12 months.
3DOSAGE FORMS AND STRENGTHS
PROPECIA tablets (1 mg) are tan, octagonal, film-coated convex tablets with "stylized P" logo on one side and PROPECIA on the other.
4CONTRAINDICATIONS
PROPECIA is contraindicated in the following:
- Pregnancy. Finasteride use is contraindicated in women when they are or may potentially be pregnant. Because of the ability of Type II 5α-reductase inhibitors to inhibit the conversion of testosterone to 5α-dihydrotestosterone (DHT), finasteride may cause abnormalities of the external genitalia of a male fetus of a pregnant woman who receives finasteride. If this drug is used during pregnancy, or if pregnancy occurs while taking this drug, the pregnant woman should be apprised of the potential hazard to the male fetus.
- Hypersensitivity to any component of this medication.
5OVERDOSAGE
In clinical studies, single doses of finasteride up to 400 mg and multiple doses of finasteride up to 80 mg/day for three months did not result in adverse reactions. Until further experience is obtained, no specific treatment for an overdose with finasteride can be recommended.
Significant lethality was observed in male and female mice at single oral doses of 1500 mg/m
6DESCRIPTION
PROPECIA (finasteride) tablets contain finasteride as the active ingredient. Finasteride, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT).
The chemical name of finasteride is
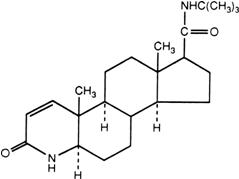
Finasteride is a white crystalline powder with a melting point near 250°C. It is freely soluble in chloroform and in lower alcohol solvents but is practically insoluble in water.
PROPECIA (finasteride) tablets are film-coated tablets for oral administration. Each tablet contains 1 mg of finasteride and the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate, hydroxypropyl methylcellulose, hydroxypropyl cellulose, titanium dioxide, magnesium stearate, talc, docusate sodium, yellow ferric oxide, and red ferric oxide.
7HOW SUPPLIED/STORAGE AND HANDLING
PROPECIA tablets, 1 mg, are tan, octagonal, film-coated convex tablets with "stylized P" logo on one side and PROPECIA on the other. They are supplied as follows:
- NDC 78206-152-01 bottles of 30 (with desiccant)
- NDC 78206-152-02 PROPAK® bottles of 90 (with desiccant).
8PATIENT COUNSELING INFORMATION
Advise the patient to read the
Exposure of Women — Risk to Male Fetus
Physicians should inform patients that women who are pregnant or may potentially be pregnant should not handle crushed or broken PROPECIA tablets because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus. PROPECIA tablets are coated and will prevent contact with the active ingredient during normal handling, provided that the tablets have not been broken or crushed. If a woman who is pregnant or may potentially be pregnant comes in contact with crushed or broken PROPECIA tablets, the contact area should be washed immediately with soap and water
Increased Risk of High-Grade Prostate Cancer
Patients should be informed that there was an increase in high-grade prostate cancer in men treated with 5α-reductase inhibitors indicated for BPH treatment, compared to those treated with placebo in studies looking at the use of these drugs to prevent prostate cancer
Sexual Adverse events
Physicians should inform the patients that PROPECIA may cause symptoms of sexual dysfunction such as decreased libido, erectile dysfunction, and ejaculation disorder, including decreased ejaculate volume.
Additional Instructions
Physicians should instruct their patients to promptly report any changes in their breasts such as lumps, pain or nipple discharge. Breast changes including breast enlargement, tenderness and neoplasm have been reported
Physicians should instruct their patients to read the patient package insert before starting therapy with PROPECIA and to read it again each time the prescription is renewed so that they are aware of current information for patients regarding PROPECIA.
9PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label
NDC 78206-152-01
Propecia
Each tablet contains 1 mg finasteride.
Rx only
30 Tablets