Hemlibra
What is Hemlibra (Emicizumab)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of the aPCC-emicizumab safety study is to investigate the hemostatic efficacy as measured by thrombin generation, of a low personalized dose of aPCC (FEIBA) in children and adults with hemophilia A and inhibitors on emicizumab prophylaxis.
Summary: This is a Phase III, multicenter, open-label clinical study designed to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of emicizumab prophylaxis in participants aged 1 month and above, who have been diagnosed with Type 3 von Willebrand disease (VWD). Participants on prior standard of care (SOC) on-demand therapy will be assessed via a randomized comparison (Arm A - emicizuma...
Summary: Recombinant factor VIII for the prevention of bleeding in patients with severe haemophilia A undergoing major surgery while receiving emicizumab prophylaxis
Related Latest Advances
Brand Information
- 12 mg/0.4 mL
- 30 mg/mL
- 60 mg/0.4 mL
- 105 mg/0.7 mL
- 150 mg/mL
- 300 mg/2 mL (150 mg/mL)
- Thrombotic Microangiopathy Associated with HEMLIBRA and aPCC
- Thromboembolism Associated with HEMLIBRA and aPCC
- Do not inject yourself or someone else unless you have been shown how to by your healthcare provider.
- Make sure the name HEMLIBRA appears on the box and vial label.
- Before opening the vial, read the vial label to make sure you have the medicine strength(s) needed to give the dose prescribed by your healthcare provider.
- Your healthcare provider will determine your dose in milliliters (mL) that you will need to give based on your body weight.
- HEMLIBRA comes in multiple strengths. Depending on your dose, you may need to use more than one vial to give your total prescribed dose.
- Check the expiration date on the box and vial label.
- Only use the vial one time. After you inject your dose, dispose of (throw away) any unused HEMLIBRA left in the vial. Do not save unused HEMLIBRA in the vial for later use.
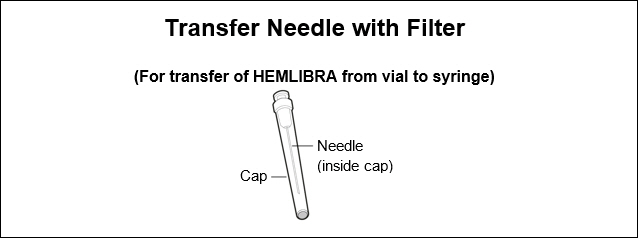
- Only use the syringes, transfer needles, and injection needles that your healthcare provider prescribes.
- Only use the syringes, transfer needles and injection needles one time. Dispose of (throw away) any used syringes and needles in a sharps disposal container.
- If your prescribed dose is more than 2 mL, you will need to give more than one injection of HEMLIBRA.
- Store HEMLIBRA in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store HEMLIBRA in the original carton to protect the vials from light.
- Do not shake HEMLIBRA.
- Take the vial out of the refrigerator 15 minutes before use and allow it to reach room temperature before preparing an injection.
- Before giving the injection, unopened vials of HEMLIBRA may be stored out of the refrigerator and then returned to the refrigerator. HEMLIBRA should not be stored out of the refrigerator:
- Collect all supplies listed below to prepare and give your injection.
- Check the expiration date on the box, on the vial label, and on the supplies listed below. Do not use if the expiration date has passed.
- Inspect the supplies for damage.
- Place the supplies on a clean, well-lit flat work surface.
- the medicine is cloudy, hazy, or colored.
- the medicine contains particles.
- the cap covering the stopper is missing.
- HEMLIBRA must not be stored
- HEMLIBRA in the syringe must be injected under the skin (subcutaneous injection) immediately.
- Dispose of (throw away) any used vial(s), needles, vial and injection needle caps, and used syringes in a sharps disposal container.
- Do not rub the injection site after an injection.
- If you see drops of blood at the injection site, you can press a sterile cotton ball or gauze over the injection site for at least 10 seconds, until bleeding has stopped.
- If you have bruising (small area of bleeding under the skin), an ice pack can also be applied with gentle pressure to the site. If bleeding does not stop, please contact your healthcare provider.
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not dispose of (throw away) any loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of (throw away) any used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
 Do not use the transfer needle to inject HEMLIBRA as this may cause harm such as pain and bleeding.
Do not use the transfer needle to inject HEMLIBRA as this may cause harm such as pain and bleeding. Do not use the transfer needle to inject HEMLIBRA as this may cause harm such as pain and bleeding.
Do not use the transfer needle to inject HEMLIBRA as this may cause harm such as pain and bleeding.