Bylvay
What is Bylvay (Odevixibat)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study will collect information from people with Progressive Familial Intrahepatic Cholestasis (PFIC) as they use odevixibat in their daily lives. Odevixibat is a medicine that helps people with PFIC, a type of rare disease that makes their liver not work well and causes itching and yellow skin. Odevixibat was first allowed to be used for PFIC in babies older than 6 months by the European Medi...
Summary: The participants of this study will be of any age who are exposed to at least 1 dose of odevixibat at any time during pregnancy (from 1 day prior to conception to pregnancy outcome) and/or at any time during lactation (up to 12 months of infant age or weaning, whichever comes first. This study will collect data obtained via a variety of sources, including enrolled pregnant or lactating participant...
Summary: This study will collect information from patients with Alagille syndrome (ALGS) as they use odevixibat (Bylvay) in their daily lives. Odevixibat is a medicine that helps patients with ALGS, a rare disease that harms their liver and causes itching. The main aim of this study is to observe the long-term, everyday effectiveness and safety of the drug odevixibat in patients with ALGS who are receiving...
Related Latest Advances
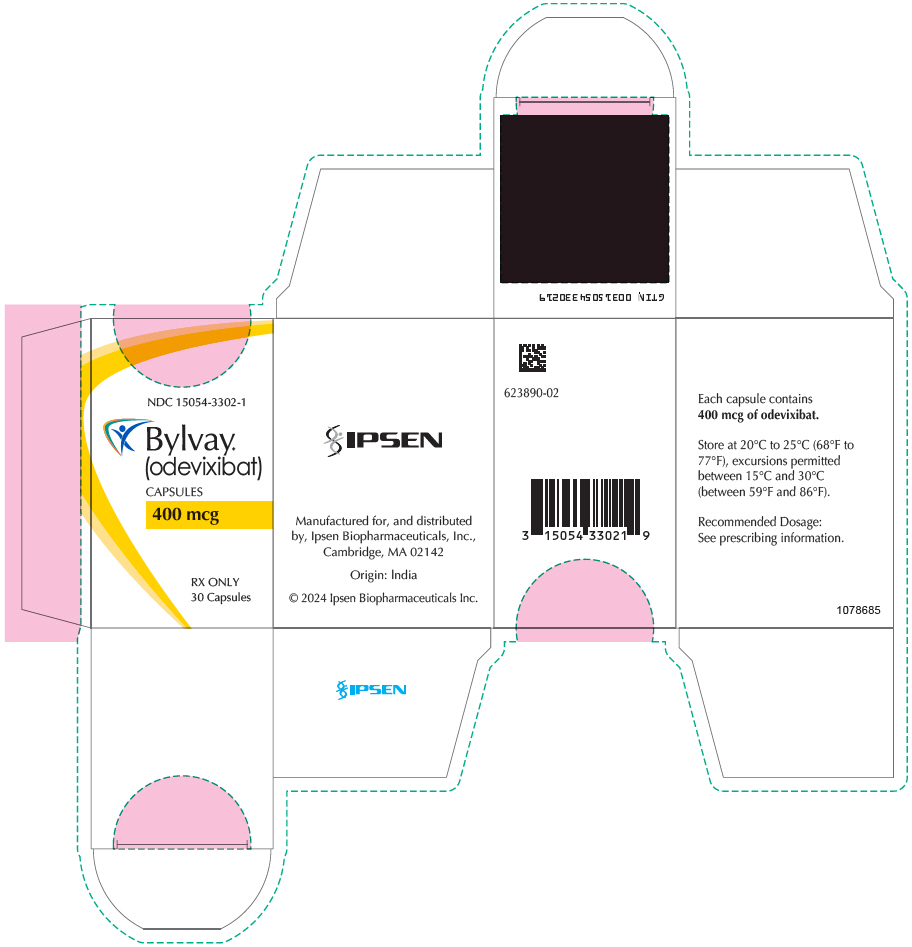
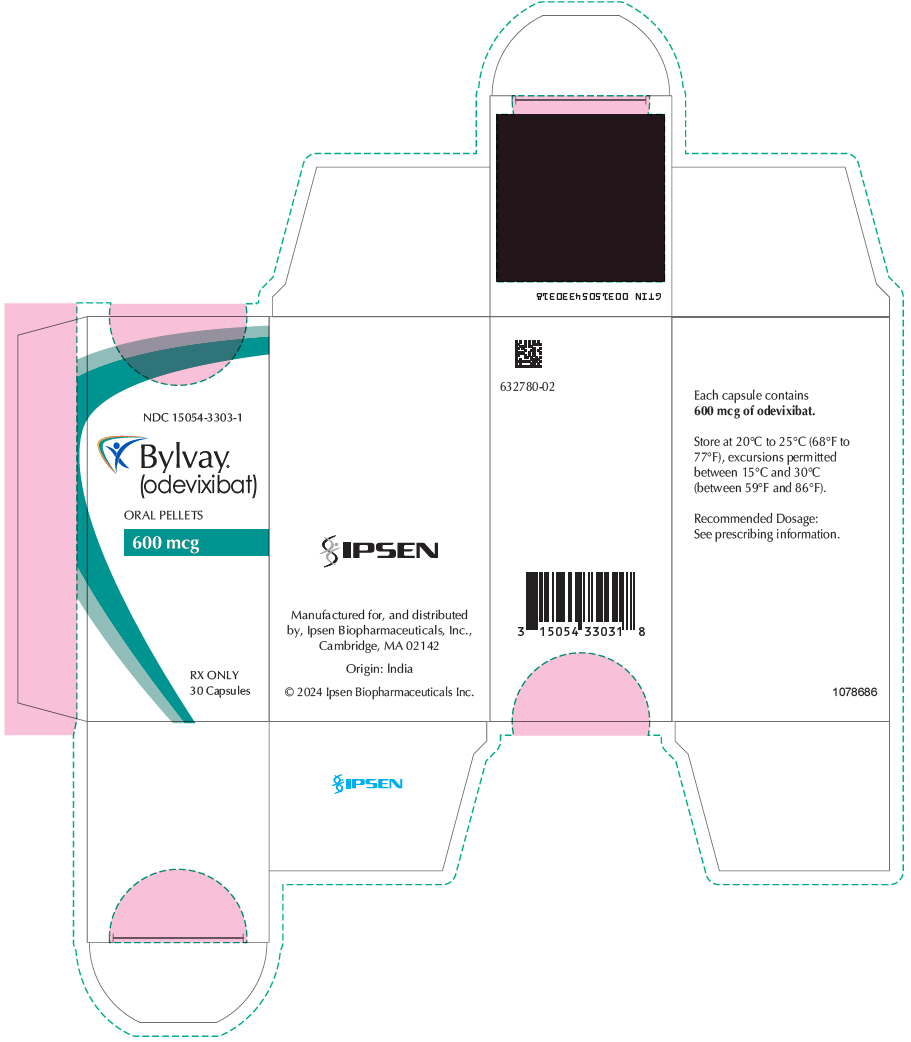
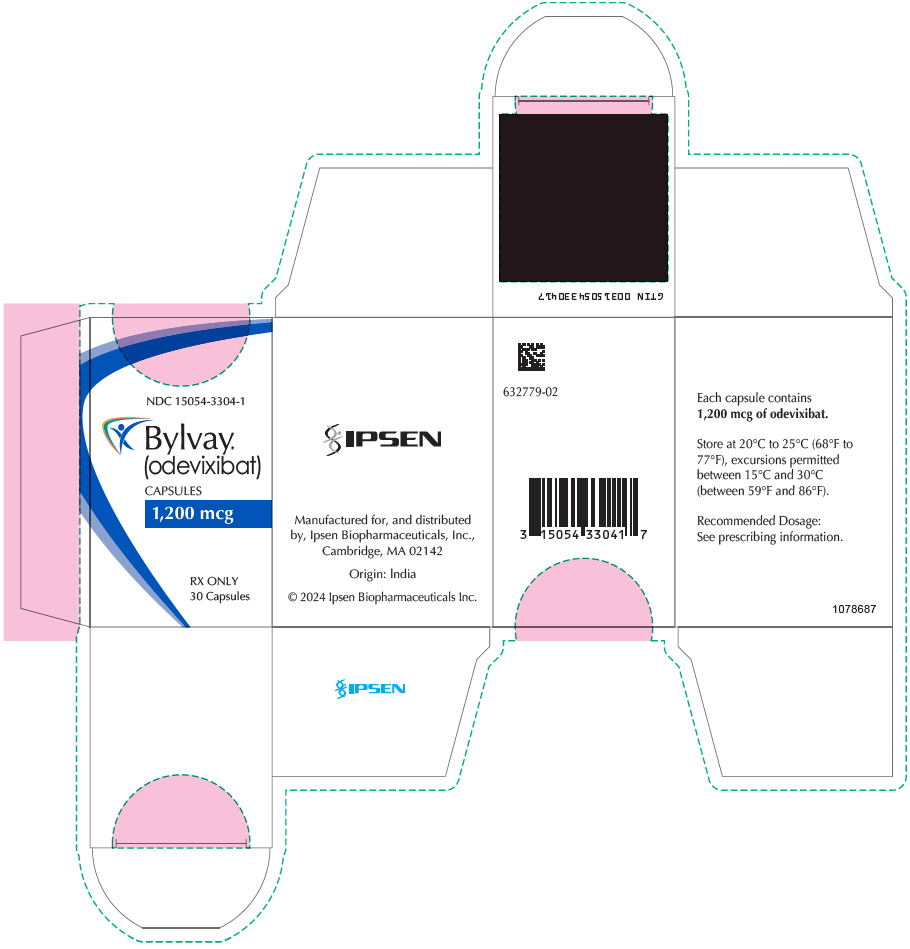
Brand Information
- Oral Pellets:
- Capsules:
- Hepatotoxicity [
- Diarrhea
- Fat-Soluble Vitamin Deficiency
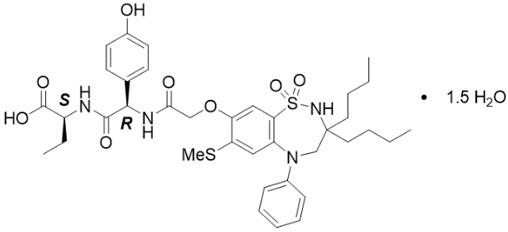
- Give BYLVAY
- Mix BYLVAY in a small amount of soft food (up to 2 tablespoons [30 mL]), such as apple sauce, oatmeal, banana or carrot puree, chocolate or rice pudding, in a bowl. You may also mix BYLVAY with an age-appropriate liquid and give through an oral syringe.
- If your child is taking bile acid binding resins (for example, cholestyramine, colestipol), give them BYLVAY at least 4 hours before or 4 hours after they take the bile acid binding resin.
- The shell containing Oral Pellets are to be opened and sprinkled.
- Mix the contents of the Oral Pellets with soft food as shown in
- The shell containing the Oral Pellets are to be opened.
- Mix the Oral Pellets with liquid as shown in
- Dispose of (throw away) the emptied shells.
- Take BYLVAY Capsules
- For children unable to swallow BYLVAY Capsules whole, follow instructions under