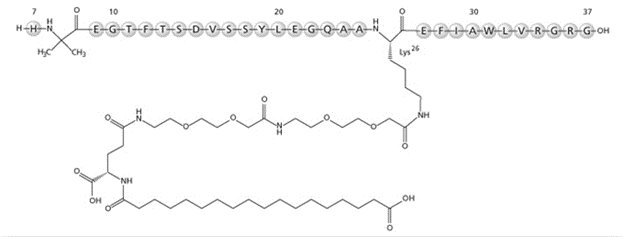
Semaglutide
What is Ozempic (Semaglutide)?
Approved To Treat
Related Clinical Trials
Summary: This phase II trial compares the effect of time-restricted eating (TRE) and glucagon-like peptide-1 (GLP1) receptor agonists (RA), semaglutide and tirzepatide, to an American Heart Association (AHA) heart healthy diet (HHD) intervention on heart and blood vessel health (cardiovascular system) and how the body processes food for energy (metabolic system) in prostate cancer patients undergoing andro...
Summary: This research study is studying WeDosify, an interactive web-based Clinical Decision Support tool that assists healthcare professionals in managing adults with excess weight or obesity using a GLP-1 drug treatment such as semaglutide.
Summary: The researchers are doing this study is to find out whether tirzepatide and semaglutide are practical (feasible) for weight management and blood sugar control for endometrial cancer patients undergoing chemotherapy. The researchers will also look at participants' experience with the study drug, the safety of taking the study drug while receiving chemotherapy, and changes in weight, body fat compos...
Related Latest Advances
Brand Information
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether OZEMPIC causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- OZEMPIC is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease.
- OZEMPIC has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis
- OZEMPIC is not indicated for use in patients with type 1 diabetes mellitus.
- A personal or family history of MTC or in patients with MEN 2
- A serious hypersensitivity reaction to semaglutide or to any of the excipients in OZEMPIC. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with OZEMPIC
- In addition to the reactions in Table 1, the following gastrointestinal adverse reactions with a frequency of <5% were associated with OZEMPIC (frequencies listed, respectively, as: placebo; 0.5 mg; 1 mg): dyspepsia (1.9%, 3.5%, 2.7%), eructation (0%, 2.7%, 1.1%), flatulence (0.8%, 0.4%, 1.5%), gastroesophageal reflux disease (0%, 1.9%, 1.5%), and gastritis (0.8%, 0.8%, 0.4%).
- This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 03/2022




