Brand Name
Brineura
Generic Name
Cerliponase
View Brand Information FDA approval date: April 27, 2017
Form: Kit
What is Brineura (Cerliponase)?
BRINEURA is indicated to slow the loss of ambulation in pediatric patients with neuronal ceroid lipofuscinosis type 2 , also known as tripeptidyl peptidase 1 deficiency. BRINEURA is a hydrolytic lysosomal N-terminal tripeptidyl peptidase indicated to slow the loss of ambulation in pediatric patients with neuronal ceroid lipofuscinosis type 2 , also known as tripeptidyl peptidase 1 deficiency.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Brineura (cerliponase alfa)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate BRINEURA in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue BRINEURA and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur
1INDICATIONS AND USAGE
BRINEURA is indicated to slow the loss of ambulation in pediatric patients with neuronal ceroid lipofuscinosis type 2 (CLN2 disease), also known as tripeptidyl peptidase 1 (TPP1) deficiency.
2DOSAGE FORMS AND STRENGTHS
Injection: BRINEURA 150 mg/5 mL (30 mg/mL) solution, two single-dose vials per carton co-packaged with Intraventricular Electrolytes Injection 5 mL in a single-dose vial. BRINEURA is a clear to slightly opalescent and colorless to pale yellow solution. Intraventricular Electrolytes is a clear to colorless solution
3CONTRAINDICATIONS
BRINEURA is contraindicated in patients with:
- any sign or symptom of acute, unresolved localized infection on or around the device insertion site (e.g., cellulitis or abscess); or suspected or confirmed CNS infection (e.g., cloudy CSF or positive CSF gram stain, or meningitis)
- any acute intraventricular access device-related complication (e.g., leakage, extravasation of fluid, or device failure)
- ventriculoperitoneal shunts.
4ADVERSE REACTIONS
The following adverse reactions are described below and elsewhere in the labeling:
- Hypersensitivity Reactions Including Anaphylaxis
- Meningitis and Other Intraventricular Access Device-Related Infections
- Intraventricular Access Device-Related Complications
- Cardiovascular Adverse Reactions
- Infusion-Associated Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of BRINEURA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Immune system disorders: Anaphylactic reaction characterized by acute pyrexia, respiratory distress (bronchospasm, hypoxemia, perioral cyanosis), tachycardia, hypotension, diarrhea, and rash [see .
- Infections and infestations: Bacterial meningitis [see .
5DESCRIPTION
Cerliponase alfa is a purified human enzyme produced by recombinant DNA technology in a Chinese hamster ovary cell line. The active substance is a recombinant human tripeptidyl peptidase-1 (rhTPP1), a lysosomal exopeptidase. The primary activity of the mature enzyme is the cleavage of N-terminal tripeptides from a broad range of protein substrates.
Cerliponase alfa contains 544 amino acids with an average molecular mass of 59 kDa. The mature enzyme is 368 amino acids in length. There are 5 consensus N-glycosylation sites on rhTPP1 that contain high mannose, phosphorylated high mannose and complex glycosylation structures.
BRINEURA (cerliponase alfa) Injection and Intraventricular Electrolytes Injection are administered by intraventricular infusion. The solutions are sterile, nonpyrogenic, and free of foreign particulates. BRINEURA is a clear to slightly opalescent and colorless to pale yellow solution. Intraventricular Electrolytes is a clear to colorless solution.
BRINEURA and Intraventricular Electrolytes Injection are packaged in 10 mL clear Type 1 single-dose glass vials
Each vial contains: sodium: 0.76 mEq, and potassium: 0.015 mEq.
6CLINICAL STUDIES
The efficacy of BRINEURA was assessed over 96 weeks in a non-randomized single-arm dose escalation clinical study with extension in symptomatic pediatric patients with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease, confirmed by TPP1 deficiency. BRINEURA-treated patients were compared to untreated patients from a natural history cohort. The Motor domain of a CLN2 Clinical Rating Scale was used to assess disease progression. Scores ranged from 3 (grossly normal) to 0 (profoundly impaired) with unit decrements representing milestone events in the loss of motor function (ability to walk or crawl). Due to the inability to establish comparability for the CLN2 Language domain ratings between the clinical study with extension and the natural history cohort, efficacy of BRINEURA for the Language domain cannot be established.
Twenty-four patients, aged 3 to 8 years were enrolled in the BRINEURA single-arm clinical study (Trial 1, NCT01907087). Sixty-three percent of patients were female and 37% were male. Ninety-six percent of patients were White and 4% were Asian; for ethnicity, 4% identified as Hispanic/Latino, 96% as non-Hispanic/Latino. One patient withdrew after week 1 due to inability to continue with study procedures; 23 patients were treated with BRINEURA 300 mg every other week by intraventricular infusion for 48 weeks, and continued treatment during the 240-week extension period, Trial 2 (NCT02485899), for a total duration of 288 weeks, plus a 24-week safety follow-up.
In the clinical study with extension, patients were assessed for decline in the Motor domain of the CLN2 Clinical Rating Scale at 48, 72 and 96 weeks. Decline was defined as having an unreversed (sustained) 2-category decline or an unreversed score of 0 in the Motor domain of the CLN2 Clinical Rating Scale. Patients' responses to BRINEURA treatment were evaluated if at screening a combined Motor plus Language CLN2 score of less than 6 was recorded. Two patients with a combined Motor plus Language CLN2 score of 6 were excluded from the analyses; they maintained that score throughout the study period. The patient who terminated early was analyzed as having a decline at the time of termination. Data used in the analyses from the natural history cohort began at 36 months of age or greater and at the first time a Motor plus Language CLN2 score less than 6 was recorded.
Motor scores of the 22 BRINEURA-treated patients in the clinical study with extension were compared to scores of the independent natural history cohort that included 42 untreated patients who satisfied inclusion criteria for the clinical study. The results of logistic modeling with covariates (screening age, screening motor score, genotype: 0 key mutations (yes/no)), demonstrated the odds of BRINEURA-treated patients not having a decline by 96 weeks were 13 times the odds of natural history cohort patients not having a decline (Odds Ratio (95% CI): 13.1 (1.2, 146.9)).
7HOW SUPPLIED/STORAGE AND HANDLING
BRINEURA is supplied as a sterile, clear to slightly opalescent and colorless to pale yellow solution for intraventricular infusion and Intraventricular Electrolytes Injection is supplied as a clear to colorless solution for intraventricular infusion; both are included in package 1 of 2. The Administration Kit for use with BRINEURA is supplied separately as package 2 of 2
8PATIENT COUNSELING INFORMATION
- Hypersensitivity Reactions Including Anaphylaxis and Infusion-Associated Reactions (IARs)
- Intraventricular Access Device-Related Infections
- Cardiovascular Adverse Reactions
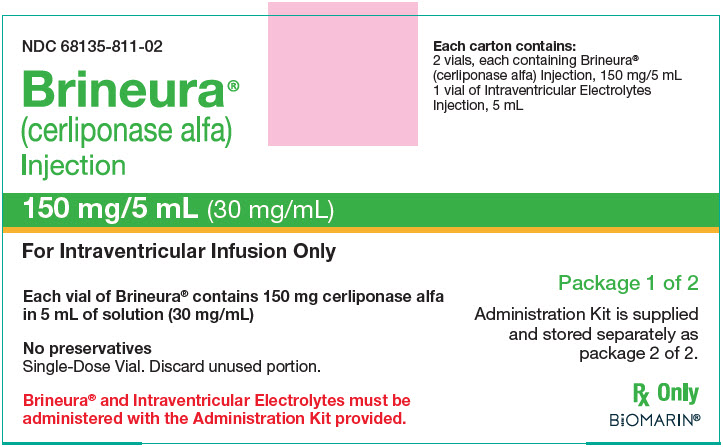
9PRINCIPAL DISPLAY PANEL - Kit Carton - Package 1
NDC 68135-811-02
Brineura
150 mg/5 mL (30 mg/mL)
For Intraventricular Infusion Only
Each vial of Brineura
No preservatives
Brineura
Each carton contains:
Package 1 of 2
Administration Kit is supplied
Rx Only
BIOMARIN
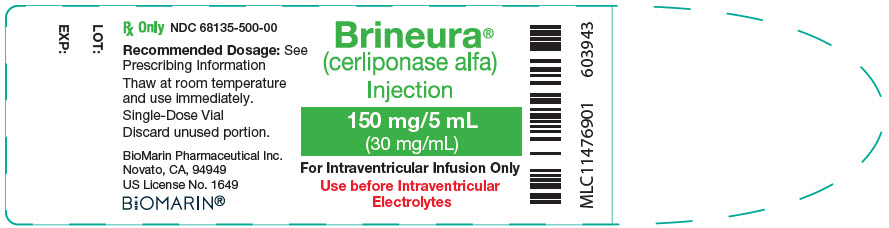
10PRINCIPAL DISPLAY PANEL - 150 mg/5 mL Vial Label
Brineura
150 mg/5 mL
For Intraventricular Infusion Only
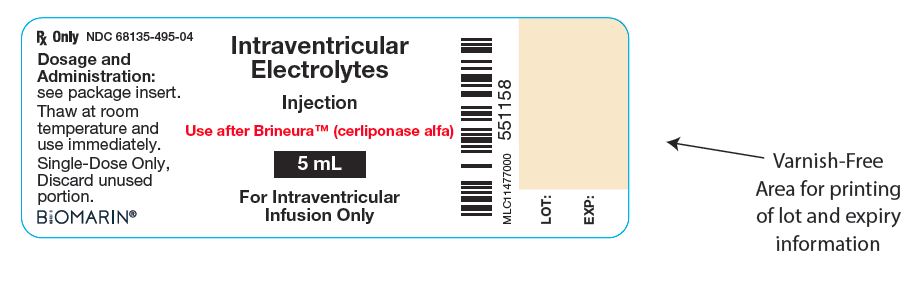
11PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
Intraventricular
Injection
Use after Brineura
5 mL
For Intraventricular
12PRINCIPAL DISPLAY PANEL - Kit Carton - Package 2
Administration Kit for use with Brineura
Recommended Dosage: See Prescribing Information
Contents of Administration Kit:
- Two single-use 20-mL syringes
- Two single-use syringe needles, 21 G, 25.4 mm
- One single-use extension line
- One single-use infusion set with 0.2 micron inline filter
- One single-use port needle, 22 G, 16 mm
Package 2 of 2
Brineura
Caution:
- Do not use if the seals or package are damaged.
- Do not resterilize.
- Single use only. Do not reuse.
- Do not freeze.
- Store in original package separately from Brineura
NOTE:
- This Administration Kit has no components made of natural latex rubber.
- Individual devices are provided in separate sterile packaging.
- Expiration dates may vary for each device included.
Manufactured for
Rx Only
BIOMARIN


