Apixaban
What is Eliquis (Apixaban)?
Approved To Treat
Related Clinical Trials
Summary: This study is researching experimental drugs called REGN7508 and REGN9933. The study is focused on participants who have atrial fibrillation, which means that the heart beats too fast and unevenly. REGN7508 and REGN9933 are designed to help stop blood clots forming in patients with atrial fibrillation. The aim of the study is to see how well REGN7508 and REGN9933 work in patients that get medicine...
Summary: This study is a prospective, multicenter, randomized controlled trial of the FlowTriever System plus anticoagulation compared to anticoagulation alone for intermediate-risk acute PE.
Summary: This study investigates the efficacy and safety of direct oral anticoagulants (DOACs) in comparison with standard low-molecular-weight heparin (LMWH) for the prevention of venous thromboembolism in patients with hematological malignancies. Eligible participants will be randomized to receive reduced-dose apixaban, reduced-dose rivaroxaban, or standard-dose LMWH. The primary objective is to evaluate...
Related Latest Advances
Brand Information
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- a history of traumatic or repeated epidural or spinal punctures
- a history of spinal deformity or spinal surgery
- optimal timing between the administration of ELIQUIS and neuraxial procedures is not known
- 2.5 mg, yellow, round, biconvex, film-coated tablets with “893” debossed on one side and “2½” on the other side.
- 5 mg, pink, oval-shaped, biconvex, film-coated tablets with “894” debossed on one side and “5” on the other side.
- Active pathological bleeding
- Severe hypersensitivity reaction to ELIQUIS (e.g., anaphylactic reactions)
- Increased Risk of Thrombotic Events After Premature Discontinuation
- Bleeding
- Spinal/Epidural Anesthesia or Puncture
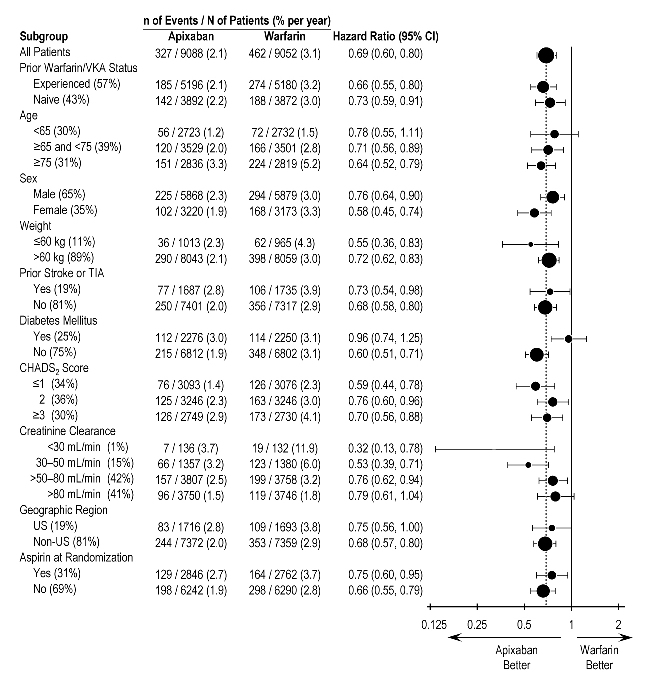
- * Bleeding events within each subcategory were counted once per subject, but subjects may have contributed events to multiple endpoints. Bleeding events were counted during treatment or within 2 days of stopping study treatment (on-treatment period).
- Not to discontinue ELIQUIS without talking to their physician first.
- That it might take longer than usual for bleeding to stop, and they may bruise or bleed more easily when treated with ELIQUIS. Advise patients about how to recognize bleeding or symptoms of hypovolemia and of the urgent need to report any unusual bleeding to their physician.
- To tell their physicians and dentists they are taking ELIQUIS, and/or any other product known to affect bleeding (including nonprescription products, such as aspirin or NSAIDs), before any surgery or medical or dental procedure is scheduled and before any new drug is taken.
- If the patient is having neuraxial anesthesia or spinal puncture, inform the patient to watch for signs and symptoms of spinal or epidural hematomas
- To tell their physicians if they are pregnant or plan to become pregnant or are breastfeeding or intend to breastfeed during treatment with ELIQUIS
- How to take ELIQUIS if they cannot swallow, or require a nasogastric tube
- What to do if a dose is missed
(apixaban)
tablets
- For people taking ELIQUIS for atrial fibrillation: People with atrial fibrillation (a type of irregular heartbeat) are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. ELIQUIS lowers your chance of having a stroke by helping to prevent clots from forming. If you stop taking ELIQUIS, you may have increased risk of forming a clot in your blood.
Do not stop taking ELIQUIS without talking to the doctor who prescribes it for you. Stopping ELIQUIS increases your risk of having a stroke.
ELIQUIS may need to be stopped, if possible, prior to surgery or a medical or dental procedure. Ask the doctor who prescribed ELIQUIS for you when you should stop taking it. Your doctor will tell you when you may start taking ELIQUIS again after your surgery or procedure. If you have to stop taking ELIQUIS, your doctor may prescribe another medicine to help prevent a blood clot from forming. - ELIQUIS can cause bleedingwhich can be serious and rarely may lead to death. This is because ELIQUIS is a blood thinner medicine that reduces blood clotting.
You may have a higher risk of bleeding if you take ELIQUIS and take other medicines that increase your risk of bleeding, including:- aspirin or aspirin-containing products
- long-term (chronic) use of nonsteroidal anti-inflammatory drugs (NSAIDs)
- warfarin sodium (COUMADIN
- any medicine that contains heparin
- selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs)
- other medicines to help prevent or treat blood clots
- you may bruise more easily
- it may take longer than usual for any bleeding to stop
- unexpected bleeding, or bleeding that lasts a long time, such as:
- bleeding that is severe or you cannot control
- red, pink, or brown urine
- red or black stools (looks like tar)
- cough up blood or blood clots
- vomit blood or your vomit looks like coffee grounds
- unexpected pain, swelling, or joint pain
- headaches, feeling dizzy or weak
- ELIQUIS is not for patients with artificial heart valves.
- Spinal or epidural blood clots (hematoma).People who take a blood thinner medicine (anticoagulant) like ELIQUIS, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:
- a thin tube called an epidural catheter is placed in your back to give you certain medicine
- you take NSAIDs or a medicine to prevent blood from clotting
- you have a history of difficult or repeated epidural or spinal punctures
- you have a history of problems with your spine or have had surgery on your spine
- ELIQUIS is not for use in people with antiphospholipid syndrome (APS), especially with positive triple antibody testing, who have a history of blood clots.
- reduce the risk of stroke and blood clots in people who have atrial fibrillation.
- reduce the risk of forming a blood clot in the legs and lungs of people who have just had hip or knee replacement surgery.
- treat blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism), and reduce the risk of them occurring again.
- currently have certain types of abnormal bleeding.
- have had a serious allergic reaction to ELIQUIS. Ask your doctor if you are not sure.
- have kidney or liver problems
- have antiphospholipid syndrome
- have any other medical condition
- have ever had bleeding problems
- are pregnant or plan to become pregnant. It is not known if ELIQUIS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ELIQUIS passes into your breast milk. You and your doctor should decide if you will take ELIQUIS or breastfeed. You should not do both.
- Take ELIQUIS exactly as prescribed by your doctor.
- Take ELIQUIS twice every day with or without food.
- Do not change your dose or stop taking ELIQUIS unless your doctor tells you to.
- If you miss a dose of ELIQUIS, take it as soon as you remember. Do not take more than one dose of ELIQUIS at the same time to make up for a missed dose.
- If you have difficulty swallowing the tablet whole, talk to your doctor about other ways to take ELIQUIS.
- Your doctor will decide how long you should take ELIQUIS.
- Do not run out of ELIQUIS. Refill your prescription before you run out.When leaving the hospital following hip or knee replacement, be sure that you will have ELIQUIS available to avoid missing any doses.
- If you take too much ELIQUIS, call your doctor or go to the nearest hospital emergency room right away.
- Call your doctor or healthcare provider right away if you fall or injure yourself, especially if you hit your head. Your doctor or healthcare provider may need to check you.
- See "
- ELIQUIS can cause a skin rash or severe allergic reaction. Call your doctor or get medical help right away if you have any of the following symptoms:
COUMADIN ®is a registered trademark of Bristol-Myers Squibb Pharma Company.
Revised September 2021
NDC 71610-811-80, Bottles of 180 Tablets
NDC 71610-811-42, Bottles of 1800 Tablets
NDC 71610-811-83, Bottles of 3600 Tablets
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20250203AMH




