Wakix
What is Wakix (Pitolisant)?
Approved To Treat
Related Clinical Trials
Summary: This is a Phase 3, randomized, double-blind, placebo-controlled, multicenter, global clinical study to assess the efficacy and safety of pitolisant in patients living with Prader-Willi syndrome. The primary objective of this study is to evaluate the efficacy of pitolisant in treating excessive daytime sleepiness (EDS) in patients ≥6 years of age with Prader-Willi syndrome. Secondary objectives inc...
Summary: The WAKIX (pitolisant) Pregnancy Registry is a US-based, prospective, observational cohort study designed to evaluate the association between pitolisant exposure during pregnancy and subsequent maternal, fetal, and infant outcomes.
Summary: The purpose of this study is to assess the tolerability of HBS-201 when starting at a therapeutic dose in adult participants with narcolepsy.
Related Latest Advances
Brand Information
- treatment of excessive daytime sleepiness (EDS) or cataplexy in adult patients with narcolepsy.
- treatment of excessive daytime sleepiness (EDS) in pediatric patients 6 years of age and older with narcolepsy.
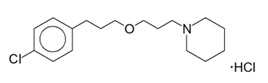
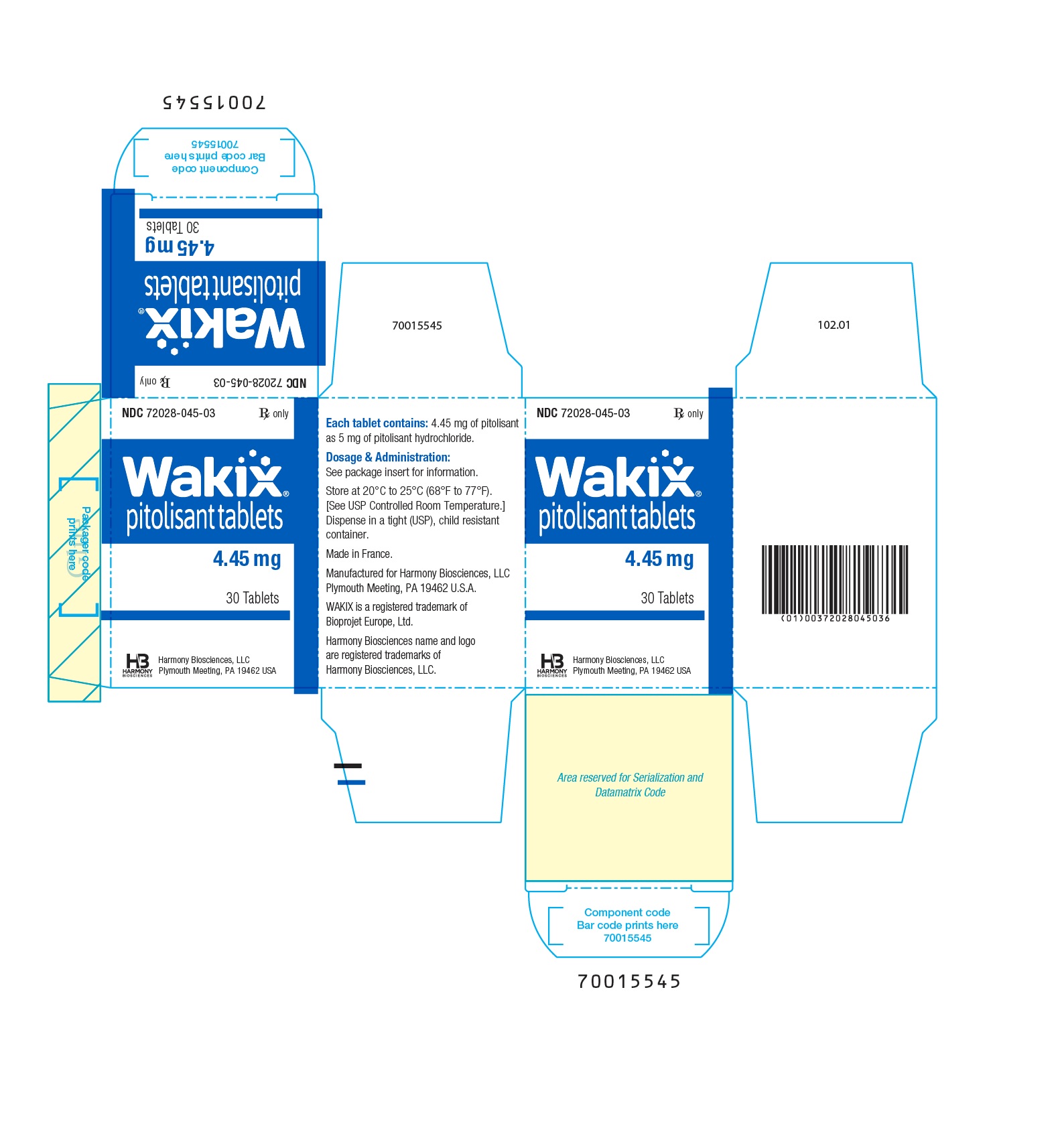
- WAKIX 4.45 mg tablets: white, round, biconvex film-coated tablet, marked with “S” on one side and plain on the other side. Each tablet contains 5 mg of pitolisant hydrochloride equivalent to 4.45 mg of pitolisant.
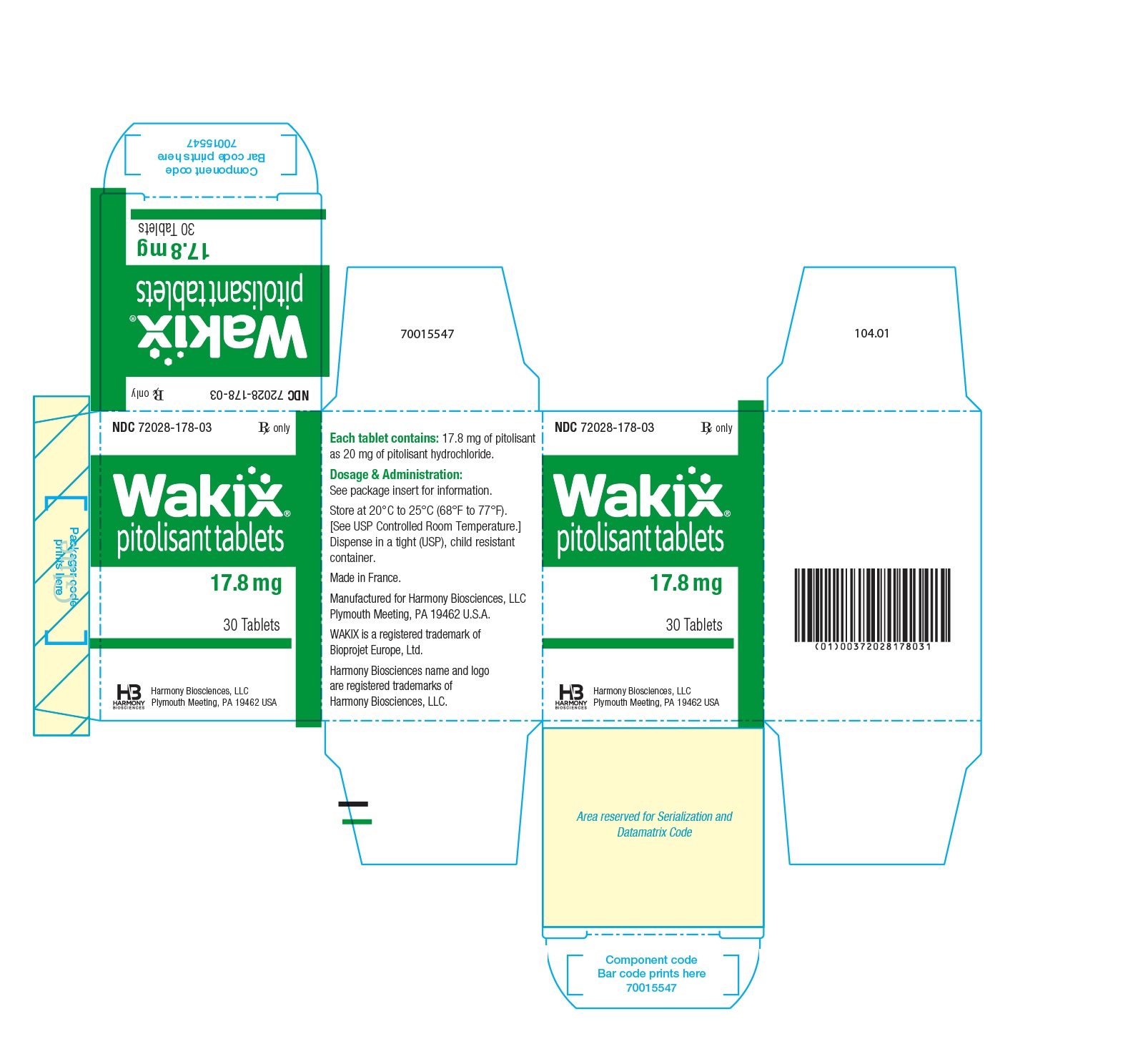
- WAKIX 17.8 mg tablets: white, round, biconvex film-coated tablet, marked with “H” on one side and plain on the other side. Each tablet contains 20 mg of pitolisant hydrochloride equivalent to 17.8 mg of pitolisant.
- known hypersensitivity to pitolisant or any component of the formulation. Anaphylaxis has been reported in patients treated with WAKIX
- severe hepatic impairment. WAKIX is extensively metabolized by the liver and there is a significant increase in WAKIX exposure in patients with moderate hepatic impairment
- QT Interval Prolongation