Tamsulosin
What is Dutasteride (Tamsulosin)?
Approved To Treat
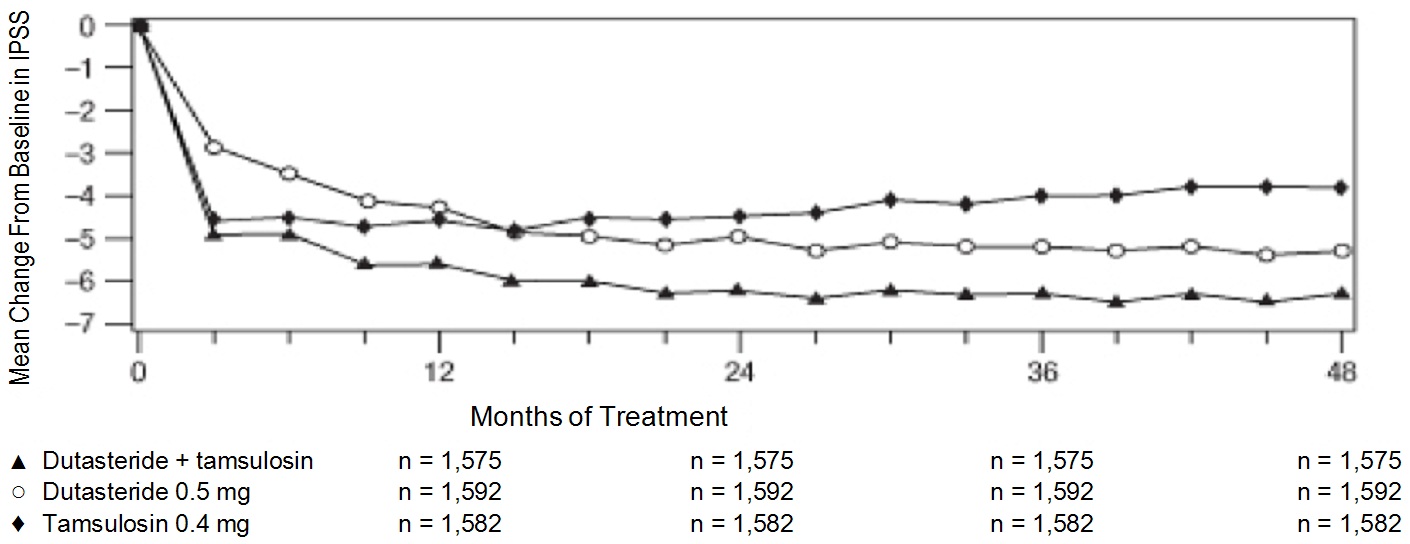
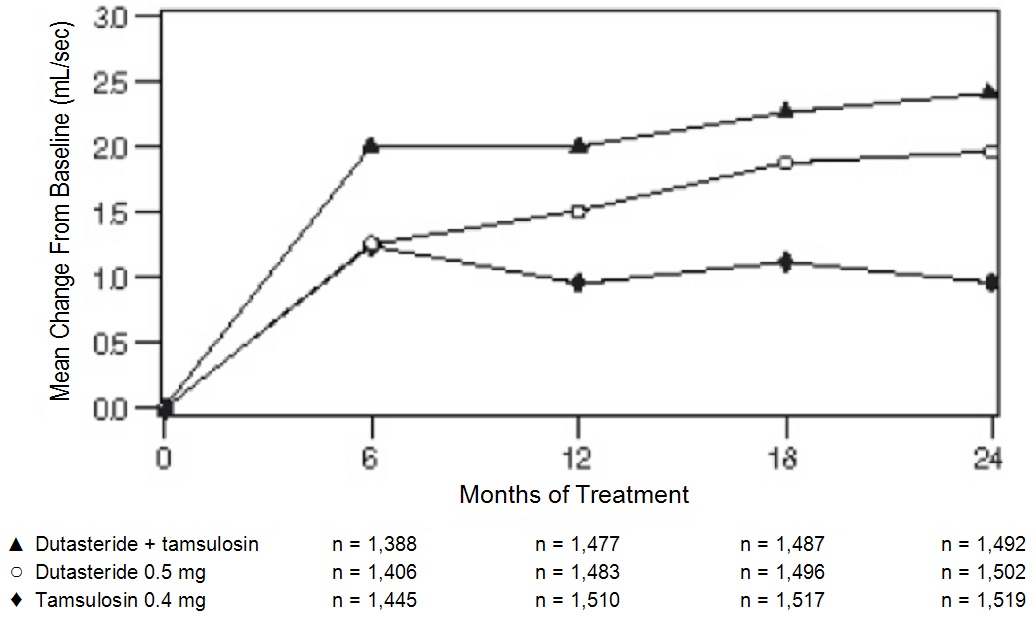
Related Clinical Trials
Summary: The aim of this study is to evaluate the bioequivalence and safety of Tamsulosin hydrochloride 0.4 mg, prolonged-release tablets (Synthon Hispania SL, Spain), compared to Omnic Ocas®, Tamsulosin hydrochloride 0.4 mg, prolonged-release film-coated tablets (Astellas Pharma Europe B.V., the Netherlands), after single dose administration in healthy adult male subjects under fasting conditions.
Summary: The purpose of this research is to see if the use of tamsulosin can decrease both the incidence and duration of urinary retention, as well as hospital length of stay following spine surgery.
Summary: A comparative study on the efficacy of Tamsulosin, Solifenacin and Mirabegron in alleviating ureteral stent-related symptoms: a randomized controlled trial.
Related Latest Advances
Brand Information
- Pregnancy. Dutasteride use is contraindicated in females who are pregnant. In animal reproduction and developmental toxicity studies, dutasteride inhibited development of male fetus external genitalia. Therefore, dutasteride and tamsulosin hydrochloride capsules may cause fetal harm when administered to a pregnant female.
- Patients with previously demonstrated, clinically significant hypersensitivity (e.g., serious skin reactions, angioedema, urticaria, pruritus, respiratory symptoms) to dutasteride, other 5-alpha-reductase inhibitors, tamsulosin, or any other component of dutasteride and tamsulosin hydrochloride capsules
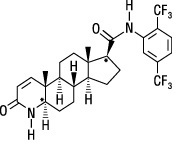
- One dutasteride oblong, opaque, yellow gelatin capsule, containing 0.5 mg of dutasteride dissolved in a mixture of butylated hydroxytoluene and mono-and di-glycerides of caprylic/capric acid. The inactive ingredients in the soft-gelatin capsule shell are ferric oxide (yellow), gelatin (from certified BSE-free bovine sources), glycerin, titanium dioxide, lecithin, medium chain triglycerides, propylene glycol, iron oxide black, polyvinyl acetate phthalate, macrogol, and ammonium hydroxide.
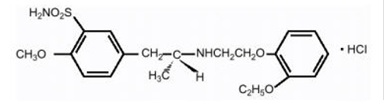
- Tamsulosin hydrochloride white to off-white pellets, containing 0.4 mg tamsulosin hydrochloride and the inactive ingredients: methacrylic acid copolymer, sugar sphere, ethylcellulose, polyethylene glycol, triethyl citrate and talc.
- pregnant or may be pregnant. Dutasteride and tamsulosin hydrochloride capsules may harm your unborn baby. Pregnant females should not touch dutasteride and tamsulosin hydrochloride capsules. If a female who is pregnant with a male baby gets enough dutasteride and tamsulosin hydrochloride capsules in her body by swallowing or touching dutasteride and tamsulosin hydrochloride capsules, the male baby may be born with sex organs that are not normal. If a pregnant female comes in contact with leaking dutasteride and tamsulosin hydrochloride capsules, the contact area should be washed immediately with soap and water.
- allergic to dutasteride, tamsulosin, or any of the ingredients in dutasteride and tamsulosin hydrochloride capsules. See the end of this leaflet for a complete list of ingredients in dutasteride and tamsulosin hydrochloride capsules.
- taking another medicine that contains an alpha-blocker.
- allergic to other 5-alpha-reductase inhibitors, for example, PROSCAR (finasteride) tablets.
- have a history of low blood pressure
- take medicines to treat high blood pressure
- plan to have cataract or glaucoma surgery
- have liver problems
- are allergic to sulfa medications
- have any other medical conditions
- Take dutasteride and tamsulosin hydrochloride capsules exactly as your healthcare provider tells you to take it.
- Swallow dutasteride and tamsulosin hydrochloride capsules whole. Do not crush, chew, or open dutasteride and tamsulosin hydrochloride capsules because the contents of the capsule may irritate your lips, mouth, or throat.
- Take your dutasteride and tamsulosin hydrochloride capsules 1 time each day, about 30 minutes after the same meal every day. For example, you may take dutasteride and tamsulosin hydrochloride capsules 30 minutes after dinner every day.
- If you miss a dose, you can take it later that same day, 30 minutes after a meal. Do not take 2 dutasteride and tamsulosin hydrochloride capsules in the same day. If you stop or forget to take dutasteride and tamsulosin hydrochloride capsules for several days, talk with your healthcare provider before starting again.
- If you take too much dutasteride and tamsulosin hydrochloride capsules, call your healthcare provider or go to the nearest hospital emergency room right away.
- Avoid driving, operating machinery, or other dangerous activities when starting treatment with dutasteride and tamsulosin hydrochloride capsules until you know how dutasteride and tamsulosin hydrochloride capsules affects you. Dutasteride and tamsulosin hydrochloride capsules can cause a sudden drop in your blood pressure, especially at the start of treatment. A sudden drop in blood pressure may cause you to faint, feel dizzy or lightheaded.
- You should not donate blood while taking dutasteride and tamsulosin hydrochloride capsules or for 6 months after you have stopped dutasteride and tamsulosin hydrochloride capsules. This is important to prevent pregnant females from receiving dutasteride and tamsulosin hydrochloride capsules through blood transfusions.
- Decreased blood pressure.Dutasteride and tamsulosin hydrochloride capsules may cause a sudden drop in your blood pressure upon standing from a sitting or lying position, especially at the start of treatment. Symptoms of low blood pressure may include:
- fainting
- dizziness
- feeling lightheaded
- Rare and serious allergic reactions, including:
- swelling of your face, tongue, or throat
- difficulty breathing
- serious skin reactions, such as skin peeling
- Higher chance of a more serious form of prostate cancer.
- Eye problems during cataract or glaucoma surgery.During cataract or glaucoma surgery, a condition called Intraoperative Floppy Iris Syndrome (IFIS) can happen if you take or have taken dutasteride and tamsulosin hydrochloride capsules in the past. If you need to have cataract or glaucoma surgery, tell your surgeon if you take or have taken dutasteride and tamsulosin hydrochloride capsules.
- A painful erection that will not go away.Rarely, dutasteride and tamsulosin hydrochloride capsules can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, there could be lasting damage to your penis, including not being able to have an erection.
- ejaculation problems*
- trouble getting or keeping an erection (impotence)*
- a decrease in sex drive (libido)*
- dizziness
- enlarged or painful breasts. If you notice breast lumps or nipple discharge, you should talk to your healthcare provider.
- runny nose
- Store dutasteride and tamsulosin hydrochloride capsules at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- Dutasteride and tamsulosin hydrochloride capsules may become deformed and/or discolored if kept at high temperatures.
- Do not use or touch dutasteride and tamsulosin hydrochloride capsules if your capsules are deformed, discolored, or leaking.
- Safely throw away medicine that is no longer needed.
