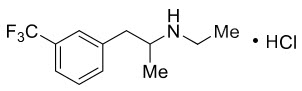
Fintepla
What is Fintepla (Fenfluramine)?
Approved To Treat
Related Clinical Trials
Summary: Dravet syndrome is a genetic epilepsy associated with pathogenic variants in SCN1A that codes for Nav1.1, a protein necessary for sodium channels. Children with Dravet syndrome classically present in the first year of life with prolonged seizures, often hemiclonic and in the setting of fever or temperature changes such as getting in or out of bath water. Many anti-seizure medications are sodium ch...
Summary: This study is a pilot non-controlled clinical trial with adjunctive fenfluramine for the treatment of five different types of developmental and epileptic encephalopathies (DEEs) focused on epileptic and non-epileptic outcomes: SYNGAP1 and STXBP1 encephalopathies, inv-dup(15) encephalopathy, multifocal or bilateral malformations of cortical development, and continuous spikes and waves during sleep....
Summary: This is a phase II clinical trial in which children with refractory infantile spasms (also called epileptic spasms or West syndrome) will be treated with fenfluramine, to evaluate efficacy, safety, and tolerability. Patients with infantile spasms that have not responded to treatment with vigabatrin and ACTH we will be invited to participate. Study participants will undergo baseline video-EEG, rece...
Related Latest Advances
Brand Information
- Hypersensitivity to fenfluramine or any of the excipients in FINTEPLA
- Concomitant use, or within 14 days of the administration, of monoamine oxidase inhibitors because of an increased risk of serotonin syndrome
- Valvular Heart Disease and Pulmonary Arterial Hypertension
- Decreased Appetite and Decreased Weight
- Somnolence, Sedation, and Lethargy
- Suicidal Behavior and Ideation
- Withdrawal of Antiepileptic Drugs
- Serotonin Syndrome
- Increase in Blood Pressure
- Glaucoma
- 1 bottle of FINTEPLA oral solution (2.2 mg/mL)
- 2 reusable oral syringes
- FINTEPLA is an
- If you have questions about how to prepare or give FINTEPLA, contact your healthcare provider or call your pharmacist.
- Always use the oral syringes provided with FINTEPLA to make sure the right dose is given. If you need a new syringe contact your pharmacist.
- Store FINTEPLA at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze.
- Keep the cap tightly closed and the bottle upright.
- Store the FINTEPLA bottle and syringe together in a clean area.
- Throw away (discard) any unused FINTEPLA 3 months after first opening the bottle
- Keep FINTEPLA and all medicines out of the reach of children.