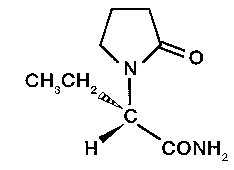
Levetiracetam
What is Keppra (Levetiracetam)?
Approved To Treat
Related Clinical Trials
Summary: This clinical trial will test whether AGB101 (low-dose levetiracetam, 220 mg, extended release tablet) can improve symptoms of psychosis in Parkinson's disease. Participants will be asked to complete up to 5 in-person study visits over approximately 20 weeks. Participants will receive both AGB101 and a placebo to take once a day for 6 weeks, with a 4-week washout in between. Participation will als...
Summary: The purpose of this study is to develop population pharmacokinetic models for antiepileptic drugs in a pediatric population. The interest of these models is multiple: * describe the pharmacokinetics of these molecules in children and explain the inter-individual variability of concentrations through covariates such as weight, age, co-treatments, genetic polymorphisms and renal function; * estimate...
Summary: The purpose of this clinical trial is to compare drug combinations to learn which drugs work best to prevent graft-versus-host-disease (GVHD) in people who have received a stem cell transplant. The source of stem cells is from someone who is not related and has a different blood cell type than the study participant. The researchers will compare the new drug combination to a standard drug combinati...
Related Latest Advances
Brand Information
- Behavioral abnormalities and Psychotic Symptoms
- Suicidal Behavior and Ideation
- Somnolence and Fatigue
- Anaphylaxis and Angioedema
- Serious Dermatological Reactions
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Coordination Difficulties
- Hematologic Abnormalities