Topiramate
What is Qsymia (Topiramate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The goal of this randomized controlled trial is to compare preoperative intensive weight management to upfront surgery in obese patients undergoing complex abdominal wall reconstruction. The main question is will abdominal wall specific quality of life (using the HerQLes survey) for the group undergoing upfront surgery be non-inferior compared to the group in the weight management program.
Summary: This goal of this study is to compare three medications used for migraine preventive treatment. This study will compare atogepant, a newer migraine preventive medication, with two older preventive medications, topiramate and propranolol. It will be determined if one works better and is more tolerable than the others. Research participants will: * Be randomly assigned to one of the three medication...
Summary: One in 10 Veterans have an alcohol use disorder. However, few Veterans receive evidenced-based psychosocial interventions or medications to treat alcohol use disorder. Barriers to receiving these treatments include long wait times, stigma, and long distances from treatment facilities. Even fewer Veterans receive psychosocial and medication interventions together, despite clinical practice guidelin...
Related Latest Advances
Brand Information
- Adults and pediatric patients aged 12 years and older with obesity
- Adults with overweight in the presence of at least one weight-related comorbid condition
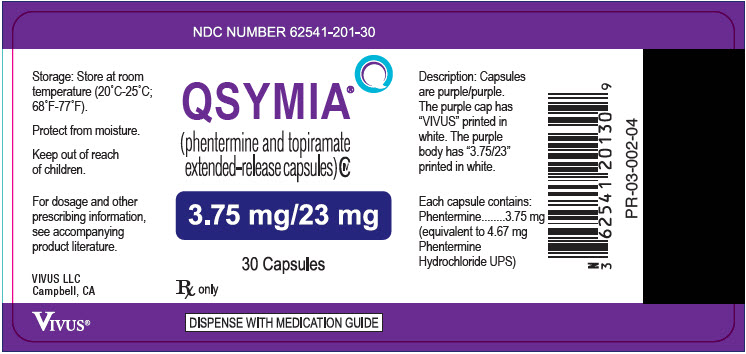
- 3.75 mg/23 mg - purple cap imprinted with VIVUS and purple body imprinted with 3.75/23
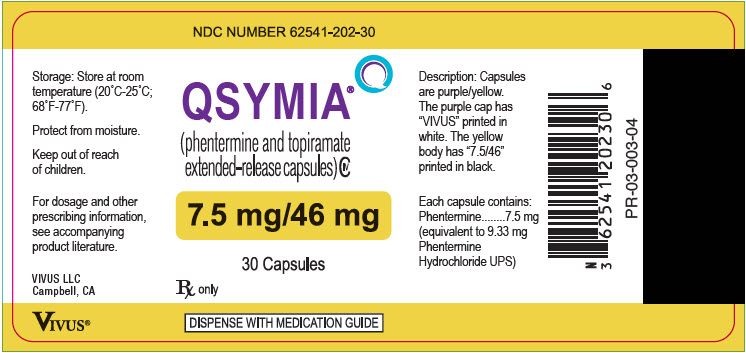
- 7.5 mg/46 mg - purple cap imprinted with VIVUS and yellow body imprinted with 7.5/46
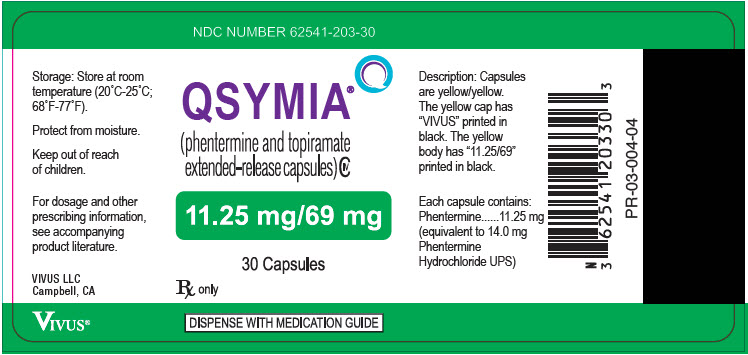
- 11.25 mg/69 mg - yellow cap imprinted with VIVUS and yellow body imprinted with 11.25/69
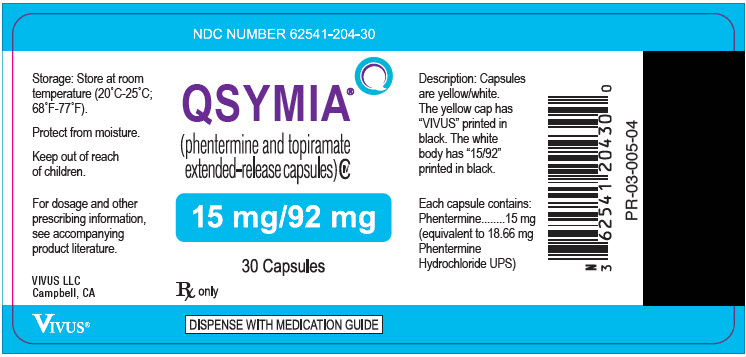
- 15 mg/92 mg - yellow cap imprinted with VIVUS and white body imprinted with 15/92
- Who are pregnant
- With glaucoma
- With hyperthyroidism
- Taking or within 14 days of stopping a monoamine oxidase inhibitors
- With known hypersensitivity to phentermine, topiramate or any of the excipients in QSYMIA, or idiosyncrasy to the sympathomimetic amines
- Embryo-Fetal Toxicity
- Suicidal Behavior and Ideation
- Risk of Ophthalmologic Adverse Reactions
- Mood and Sleep Disorders
- Cognitive Impairment
- Slowing of Linear Growth
- Metabolic Acidosis
- Decrease in Renal Function
- Risk of Seizures with Abrupt Withdrawal of QSYMIA
- Kidney Stones
- Oligohydrosis and Hyperthermia
- Hypokalemia
- Serious Skin Reactions