Qinlock
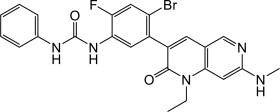
What is Qinlock (Ripretinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a Phase 1/2, multicenter, open-label (unless otherwise specified in a combination-specific module) study of inlexisertib in combination with anticancer therapies. Modules within the master protocol are defined according to different combinations of inlexisertib with other anticancer agents.
Summary: This is a prospective, single-center, observational study to explore the correlation between ripretinib exposure and the efficacy and safety in patients with advanced gastrointestinal stromal tumors
Summary: The goal of this prospective study is to learn whether there is a synergistic effect when radiotherapy is combined with ripretinib in the treatment of patients with unresectable advanced GIST. It will also learn about the safety of this regimen.The main questions it aim to answer is: Dose radiotherapy combined with ripretinib prolong the time to disease progression for patients with advanced GIST?
Related Latest Advances
Brand Information
- Palmar-Plantar Erythrodysesthesia Syndrome
- New Primary Cutaneous Malignancies
- Hypertension
- Cardiac Dysfunction
- Photosensitivity
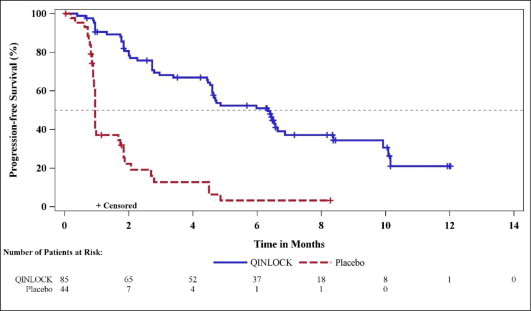
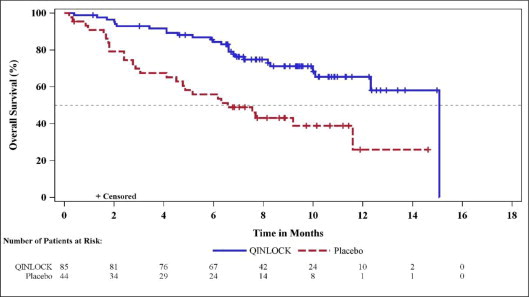
- 90-count bottles NDC 73207-101-30

