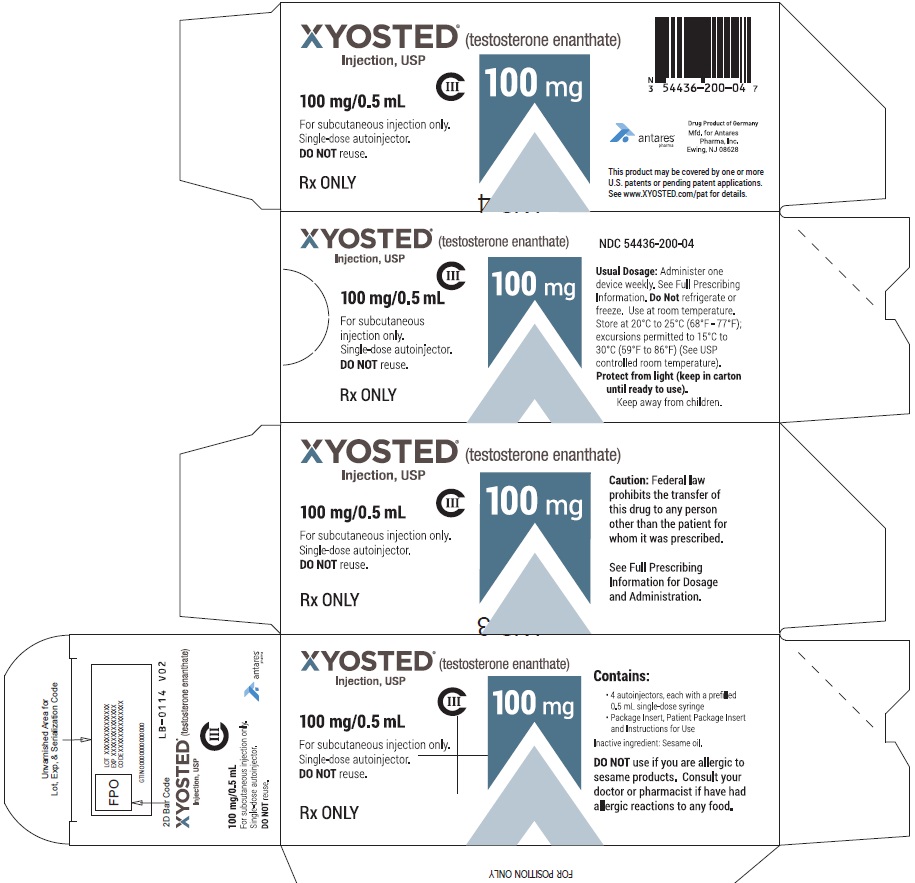
Xyosted
What is Xyosted (Enanthate)?
Approved To Treat
Related Clinical Trials
Summary: This is a 52-week open label single arm study to investigate the effects of XYOSTED, as testosterone replacement therapy, on adolescent males with either primary or secondary hypogonadism. The study aims to determine the effectiveness of XYOSTED measured by continuation or induction of puberty in addition to XYOSTED dosage, safety and testosterone levels.
Summary: The WOMBAT study will test if BAT can prolong the time it takes for nmCRPC prostate cancer to become detectable in other areas of the body (metastatic disease). Approximately 69 participants over the age of 18 with castrate resistant prostate cancer, no evidence of metastatic disease (M0) on conventional imaging (WBBS and CT scan at screening) and PSA only progression on darolutamide will be enrol...
Summary: Gender incongruence, now classified in ICD-11 as a marked and persistent incongruence between an individual's experienced gender and the gender assigned at birth, is managed in dedicated, multidisciplinary centres that coordinate psychological support with medical-surgical care. Gender-affirming hormone therapy (GAHT) is central to this care pathway. In particular, masculinising GAHT for people as...
Related Latest Advances
Brand Information
- Primary hypogonadism (congenital or acquired): testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.
- Safety and efficacy of XYOSTED in men with “age-related hypogonadism” has not been established.
- Safety and efficacy of XYOSTED in males less than 18 years old have not been established
- Men with carcinoma of the breast or known or suspected carcinoma of the prostate
- Women who are pregnant. Testosterone can cause virilization of the female fetus when administered to a pregnant woman
- Men with known hypersensitivity to XYOSTED or any of its ingredients (testosterone enanthate and sesame oil).
- Inform patients that XYOSTED can cause venous thromboembolism. Advise patients of the signs and symptoms of venous thromboembolism, which may include the following: lower limb pain, edema, or erythema; and dyspnea or chest pain. Advise patients to promptly report the signs and symptoms of venous thromboembolism, discontinue use of XYOSTED, and seek urgent medical care.
- Inform patients that XYOSTED can increase BP which can increase cardiovascular risk over time.
- Instruct patients about the importance of monitoring BP periodically while on XYOSTED. If BP increases while on XYOSTED, antihypertensive medications may need to be started, added, or adjusted to control BP, or XYOSTED may need to be discontinued.
Inform patients that treatment with androgens may lead to adverse reactions which include:
- Changes in urinary habits related to effects on prostate size, such as increased urination at night, hesitancy, urinary frequency, urinary urgency, having a urine accident, or being unable to pass urine or weak urine flow.
- Breathing disturbances that may reflect obstructive sleep apnea, including those associated with sleep or excessive daytime sleepiness.
- Too frequent or persistent erections of the penis.
- Ankle swelling that may reflect peripheral edema.
- Red blood cell count increase, PSA increase, injection site bruising, injection site bleeding.
Testosterone Enanthate Injection USP CIII
- Use XYOSTED exactly as your healthcare provider tells you to take it.
- Inject XYOSTED only 1 time each week. Do not use XYOSTED every day.
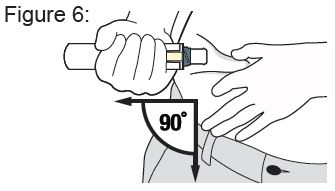
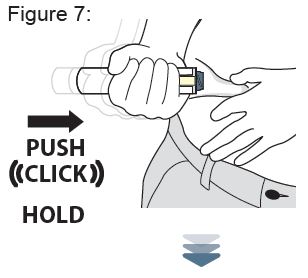
- Your healthcare provider will show you or your caregiver how to inject XYOSTED. You should not inject XYOSTED until you have been trained on the proper way to use it.
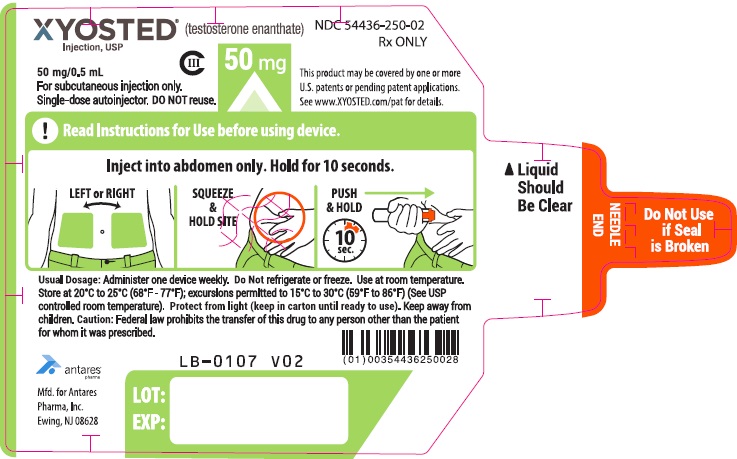
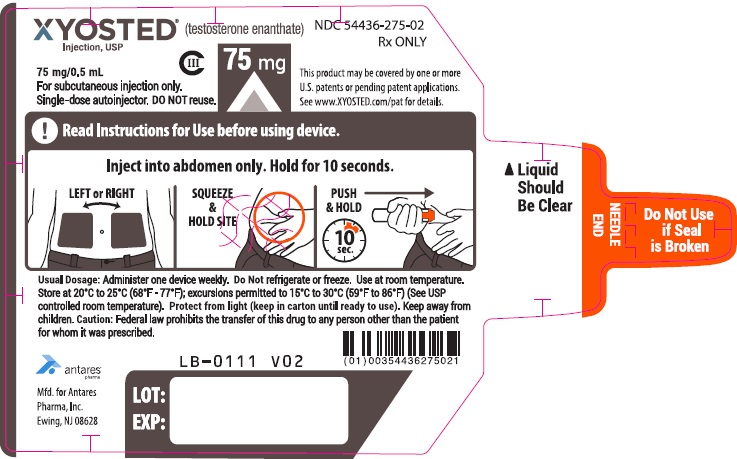
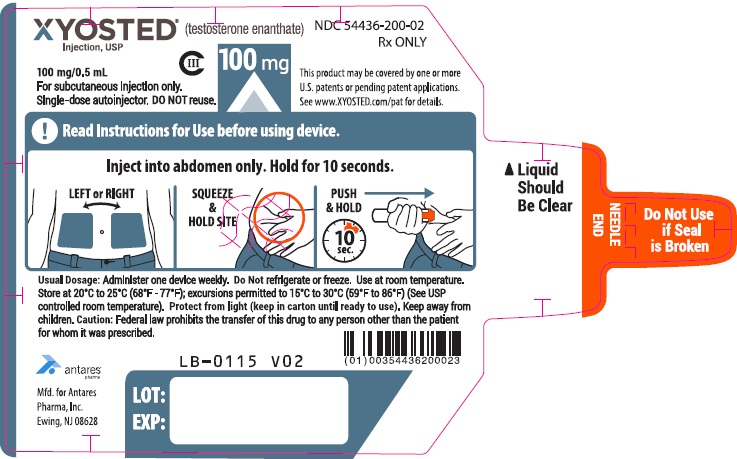
- Check XYOSTED before you inject it. XYOSTED should be clear to light yellow in color and should be free of visible particles.
- Do not use if the liquid is cloudy or if visible particles are present. You may see air bubble(s), this is normal.
- Do not use XYOSTED if the safety seal is broken or if the Auto-Injector appears broken, damaged or changed in any way.
- XYOSTED should be injected in the stomach (abdomen) area after cleaning the skin.
- Do not inject XYOSTED within 2 inches of the belly button (navel).
- Do not inject XYOSTED in any other areas of the body.
- Do not inject XYOSTED in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.
- Discard unused portion.
- 1 XYOSTED Auto-Injector
- 1 alcohol swab
- 1 cotton ball or gauze
- Do not refrigerate or freeze.
- Protect from light.
- Use at room temperature.
- Store at 68° - 77° F (20° - 25° C).
- Keep XYOSTED and all medicines away from children.
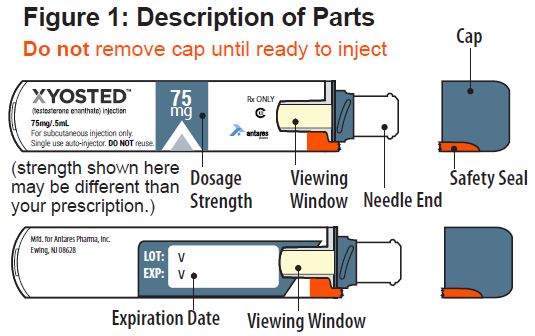
- Do not remove Cap until ready to inject.
- Inspect Auto-Injector for any visible damage.
- Check the expiration date.
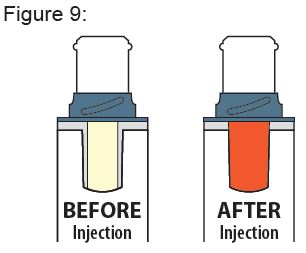
- Inspect the medicine through the Viewing Window; it should be clear to light yellow and free of visible particles (
Allow the site to dry on its own.
Do not fan or blow on the injection site.
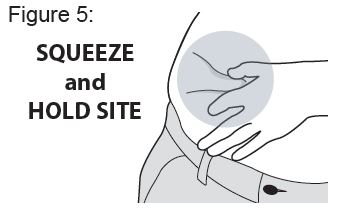
Do not touch the site again before injecting.
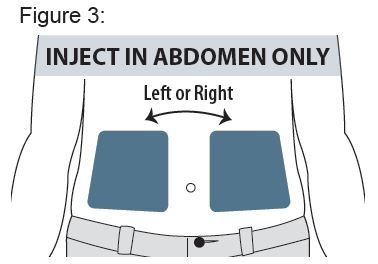
Only use the left or right side of the abdomen (belly) for injection sites.
Do not use the area within 2 inches around your navel (belly button).
See Figure 3.
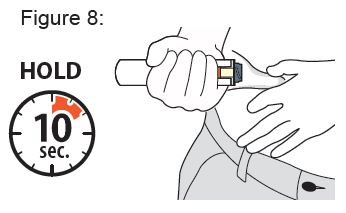
- Do not use another Auto-Injector.
- Do not attempt another injection.
- Call your healthcare provider or 1-844-996-7833 for assistance.
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container