Strensiq
What is Strensiq (Asfotase)?
For individuals born with hypophosphatasia (HPP), even simple milestones like walking, eating, or growing strong bones can be extraordinarily difficult. HPP is a rare, inherited disorder that weakens bones and teeth due to a lack of an essential enzyme that helps minerals harden the skeleton. Strensiq (asfotase alfa) is a life-changing medication developed specifically to address this root cause, offering hope where previously there were few options.
Strensiq is a recombinant enzyme replacement therapy used to treat patients with perinatal-, infantile-, or juvenile-onset hypophosphatasia. It replaces the missing or defective enzyme known as tissue-nonspecific alkaline phosphatase (TNSALP), a crucial component for healthy bone and tooth mineralization. Approved by the U.S. Food and Drug Administration (FDA) in 2015, Strensiq represents a breakthrough in the management of this rare genetic condition, helping patients achieve stronger bones, improved mobility, and better quality of life.
What does Strensiq do?
Strensiq is prescribed to treat hypophosphatasia, a condition caused by mutations in the ALPL gene that result in low activity of the alkaline phosphatase enzyme. Without this enzyme, important minerals such as calcium and phosphate cannot properly bind to the bones, leading to soft, fragile bones, poor growth, breathing difficulties, and early tooth loss.
By replacing the missing enzyme, Strensiq helps restore normal bone mineralization and supports healthy skeletal development. This, in turn, can:
- Reduce bone pain and fractures
- Improve strength and mobility
- Support normal growth in children
- Improve respiratory function in severe cases
Clinical studies have shown that children treated with Strensiq experienced significant improvements in bone structure, growth rate, and survival outcomes, compared to untreated patients (NIH, 2024). Many caregivers report that the therapy allows children to reach developmental milestones that would otherwise be impossible, such as standing and walking independently.
While Strensiq is not a cure, it offers long-term disease control and can dramatically enhance daily function and comfort in affected individuals.
How does Strensiq work?
Strensiq works by replacing the missing alkaline phosphatase enzyme in the body. In people with hypophosphatasia, this enzyme is either missing or defective, leading to a buildup of substances that prevent bones and teeth from hardening properly.
The medication contains asfotase alfa, a lab-engineered version of the natural TNSALP enzyme, which is attached to a protein fragment that helps it target the bones where it’s needed most. Once there, it breaks down excess molecules that interfere with mineral deposition, mainly inorganic pyrophosphate (PPi) allowing calcium and phosphate to bind normally to form strong, healthy bone tissue.
Clinically, this mechanism is vital because it addresses the underlying cause of HPP rather than just its symptoms. By restoring the enzyme’s function, Strensiq helps the body rebuild bone strength from within, improving skeletal structure, reducing fractures, and enhancing physical activity over time.
Strensiq side effects
Like any medication, Strensiq can cause side effects, though not everyone experiences them. Most are mild to moderate and manageable under medical supervision.
Common side effects may include:
- Redness, itching, or pain at the injection site
- Headache or fever
- Nausea or vomiting
- Localized swelling or bruising
- Cough or mild respiratory symptoms
Serious side effects (less common):
- Signs of allergic reaction, such as rash, difficulty breathing, or swelling of the face or throat
- Lipodystrophy (changes in fat distribution under the skin at injection sites)
- Calcium or phosphate imbalances in the blood
- Eye inflammation (conjunctivitis) or abnormal bone growth
Patients should inform their doctor if they experience persistent pain, severe injection reactions, or signs of hypersensitivity. In rare cases, life-threatening allergic reactions (anaphylaxis) may occur, requiring immediate emergency care.
Before starting Strensiq, patients should discuss any existing kidney, liver, or cardiovascular conditions with their healthcare provider. Ongoing monitoring helps ensure that any adverse reactions are detected early and managed appropriately.
Despite potential side effects, Strensiq’s benefits in restoring bone health and function far outweigh its risks for most patients with severe hypophosphatasia.
Strensiq dosage
Strensiq is a subcutaneous injection given multiple times weekly, with dosage varying by age, weight, and disease severity. A specialist typically initiates and supervises treatment, with caregivers trained for at-home administration at rotating sites.
Doctors monitor patients via blood tests and imaging to check enzyme activity, calcium/phosphate balance, and bone growth, ensuring treatment safety and effectiveness. Young children often show improved strength, energy, and skeletal stability within months of consistent use; long-term adherence is crucial.
No dosage adjustments exist for older adults as Strensiq is mainly for children and young adults with early-onset disease, but medical oversight is vital for all patients.
Does Strensiq have a generic version?
As of 2025, Strensiq (asfotase alfa) does not have a generic version available in the United States or internationally. It remains a brand-name biologic therapy manufactured by Alexion Pharmaceuticals. However, international versions may exist in other markets.
Strensiq, a complex biologic, is currently the sole FDA-approved treatment for hypophosphatasia. Producing generic equivalents is difficult due to its complexity, requiring rigorous FDA evaluation for biosimilars. Patients should obtain Strensiq through certified specialty pharmacies, and assistance programs are available for cost challenges.
Conclusion
Strensiq represents a groundbreaking advancement for patients living with hypophosphatasia, a condition once thought untreatable. By directly replacing the missing enzyme that causes fragile bones and poor growth, Strensiq helps rebuild skeletal strength, improve mobility, and enhance overall quality of life.
While it requires regular injections and ongoing monitoring, the benefits are profound. Many achieve previously unimaginable milestones like walking, playing, and living pain-free. Open communication with your healthcare team is vital; discuss progress, report side effects, and adhere to dosing. Under expert care, Strensiq offers strength, stability, and future hope.
References
- U.S. Food and Drug Administration (FDA). (2024). Strensiq (asfotase alfa) prescribing information. Retrieved from https://www.accessdata.fda.gov
- National Institutes of Health (NIH). (2024). Hypophosphatasia and enzyme replacement therapy overview. Retrieved from https://www.nih.gov
- Mayo Clinic. (2024). Asfotase alfa injection: Uses, side effects, and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Asfotase alfa (Strensiq): Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
Approved To Treat
Related Clinical Trials
Summary: In this prospective observational sub-study, participants with pediatric-onset hypophosphatasia (HPP) (perinatal/infantile- or juvenile-onset) of any age will be followed for a minimum of 5 years at sites in the United States and potentially 1 or 2 other countries.
Related Latest Advances
Brand Information
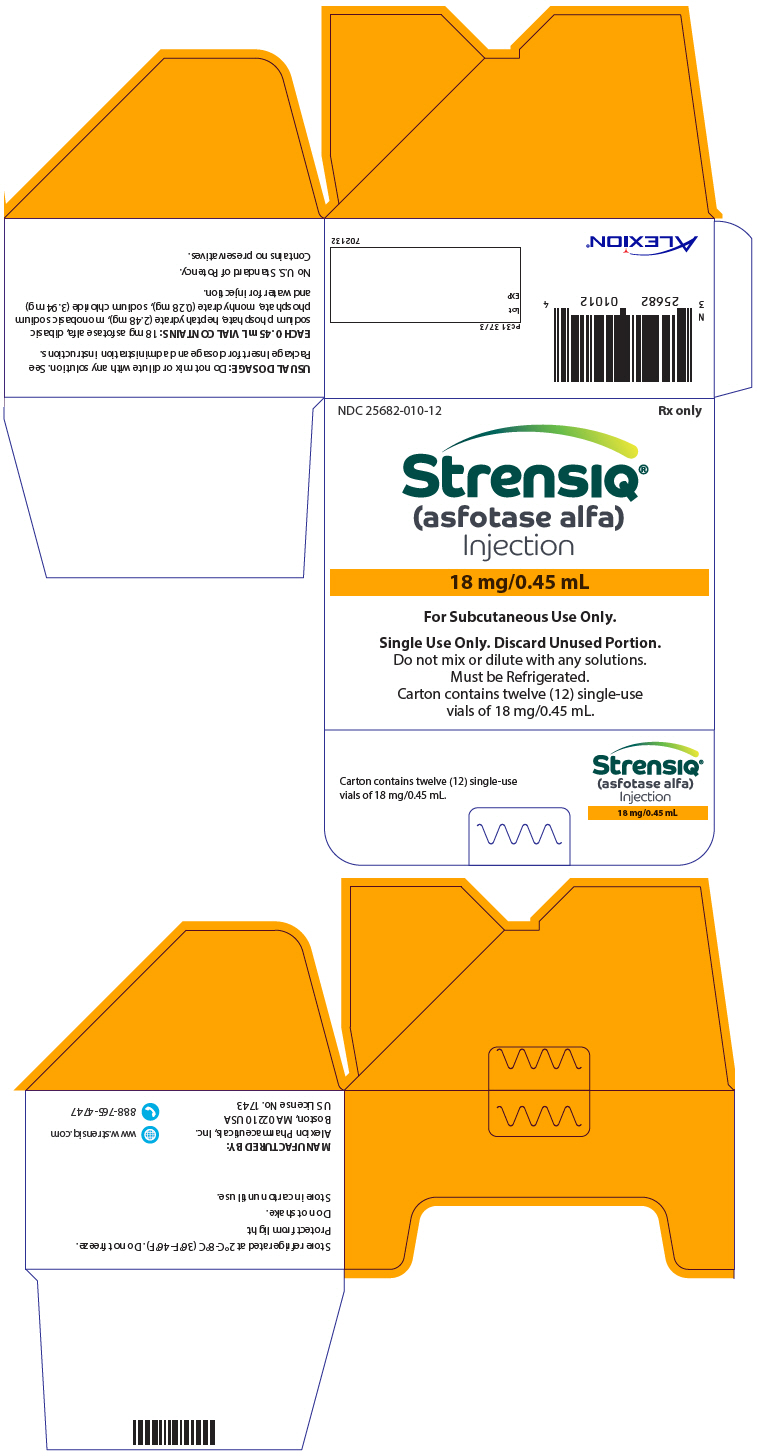
- Injection: 18 mg/0.45 mL, 28 mg/0.7 mL, 40 mg/mL, or 80 mg/0.8 mL solution in single-dose vials
- Hypersensitivity Reactions
- Lipodystrophy
- Ectopic Calcifications
- Possible Immune-Mediated Clinical Effects
- 1 or 2 STRENSIQ vial(s).
- 1 or 2 sterile disposable 1 mL syringes for injection with 25 to 29 gauge (G), ½ inch needles.
- 2 alcohol wipes
- 1 gauze or cotton ball
- a clean flat surface, like a table
- 1 sharps container for throwing away used needles and syringes. See
- Store STRENSIQ in the original carton in the refrigerator between 36°F to 46°F (2°C to 8°C) until you are ready to use it.
- Do not freeze your STRENSIQ vials. Do not use STRENSIQ if it has been frozen.
- Do not shake your STRENSIQ vials.
- Protect STRENSIQ from light until you are ready to use it.
- Do not use STRENSIQ after the expiration date printed on the carton.
- STRENSIQ vials are for 1 time use only. Throw away any unused STRENSIQ left in the vial.
- Prepare a clean flat surface, like a table or counter top.
- Remove the unopened STRENSIQ vial(s) out of the refrigerator and allow it to sit at room temperature for at least 15 to 30 minutes. Injecting STRENSIQ when cold can make the injection feel uncomfortable.
- Gather all the supplies you will need to give your STRENSIQ injection.
- Wash your hands with soap and water.
- Utilize STRENSIQ within 3 hours after removing it from the refrigerator.
- Inject STRENSIQ exactly as your healthcare provider has shown you.
- Put your used needles in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.