Brand Name
Mefloquine
View Brand InformationFDA approval date: January 06, 2004
Classification: Antimalarial
Form: Tablet
What is Mefloquine?
Treatment of Acute Malaria Infections Mefloquine Hydrochloride Tablets are indicated for the treatment of mild to moderate acute malaria caused by mefloquine-susceptible strains of P. falciparum or by Plasmodium vivax. There are insufficient clinical data to document the effect of mefloquine in malaria caused by P. ovale or P. malariae. Note: Patients with acute P. vivax malaria, treated with mefloquine, are at high risk of relapse because mefloquine does not eliminate exoerythrocytic parasites. To avoid relapse, after initial treatment of the acute infection with mefloquine, patients should subsequently be treated with an 8-aminoquinoline derivative . Prevention of Malaria Mefloquine Hydrochloride Tablets are indicated for the prophylaxis of P. falciparum and P. vivax malaria infections, including prophylaxis of chloroquine-resistant strains of P. falciparum.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Mefloquine Hydrochloride (Mefloquine Hydrochloride)
1DESCRIPTION
Mefloquine Hydrochloride Tablets USP are an antimalarial agent available as 250 mg tablets of mefloquine hydrochloride (equivalent to 228 mg of the free base) for oral administration.
Mefloquine Hydrochloride USP is a 4-quinolinemethanol derivative with the specific chemical name of (R*, S*)-(±)-α-2-piperidinyl-2,8-bis (trifluoromethyl)-4-quinolinemethanol hydrochloride. It is a 2-aryl substituted chemical structural analog of quinine. The drug is a white to almost white crystalline compound, slightly soluble in water. The structural formula is as follows:
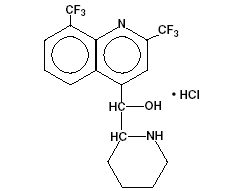
C
The inactive ingredients are colloidal silicon dioxide, corn starch, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, poloxamer and talc.
2CONTRAINDICATIONS
Use of mefloquine hydrochloride tablets is contraindicated in patients with a known hypersensitivity to mefloquine or related compounds (e.g., quinine and quinidine) or to any of the excipients contained in the formulation. Mefloquine hydrochloride tablets should not be prescribed for prophylaxis in patients with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia or other major psychiatric disorders, or with a history of convulsions.
3WARNINGS
In case of life-threatening, serious or overwhelming malaria infections due to
3.1QTc Interval Prolongation and Drug Interactions
Halofantrine should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval (see
Ketoconazole should not be administered with mefloquine or within 15 weeks of the last dose of mefloquine due to the risk of a potentially fatal prolongation of the QTc interval. Ketoconazole increases plasma concentrations and elimination half-life of mefloquine following coadministration (see
Concomitant administration of mefloquine and quinine or quinidine may produce electrocardiographic abnormalities.
3.2Psychiatric and Neurologic Adverse Reactions
Mefloquine may cause neuropsychiatric adverse reactions in adults and children. Neuropsychiatric symptoms can be difficult to identify in children. Therefore, vigilance is required to monitor for the occurrence of these symptoms, especially in nonverbal children.
3.3Psychiatric Adverse Reactions
Psychiatric symptoms ranging from anxiety, paranoia, and depression to hallucinations and psychotic behavior can occur with mefloquine use. Symptoms may occur early in the course of mefloquine use. In some cases, these symptoms have been reported to continue for months or years after mefloquine has been stopped. Cases of suicidal ideation and suicide have been reported.
Mefloquine should not be prescribed for prophylaxis in patients with active depression, generalized anxiety disorder, psychosis, or schizophrenia or other major psychiatric disorders. Mefloquine should be used with caution in patients with a previous history of depression.
During prophylactic use, the occurrence of psychiatric symptoms such as acute anxiety, depression, restlessness or confusion suggest a risk for more serious psychiatric disturbances or neurologic adverse reactions. In these cases, the drug should be discontinued and an alternative medication should be substituted.
3.4Neurologic Adverse Reactions
Neurologic symptoms such as dizziness or vertigo, tinnitus, and loss of balance have been reported. These adverse reactions may occur early in the course of mefloquine use and in some cases have been reported to continue for months or years after mefloquine has been stopped. Dizziness or vertigo, tinnitus, and loss of balance have been reported to be permanent in some cases. During prophylactic use, if neurologic symptoms occur, the drug should be discontinued and an alternative medication should be substituted.
Caution should be exercised with regard to activities requiring alertness and fine motor coordination, such as driving, piloting aircraft, operating machinery, and deep-sea diving, while symptoms persist.
Mefloquine may increase the risk of convulsions in patients with epilepsy. The drug should therefore be prescribed only for curative treatment in such patients and only if there are compelling medical reasons for its use (see
Concomitant administration of mefloquine and quinine or chloroquine may increase the risk of convulsions.
3.5Ocular Effects
Eye disorders, including but not limited to optic neuropathy and retinal disorders, have been reported during treatment with mefloquine. Any patient presenting with visual symptoms should be referred to the treating physician and an ophthalmologist as certain conditions (such as retinal disorders or optic neuropathy) may require stopping treatment with mefloquine (see
4HOW SUPPLIED
Product: 50090-2396
NDC: 50090-2396-0 10 TABLET in a BLISTER PACK / 1 in a CARTON
5ANIMAL TOXICOLOGY
Ocular lesions were observed in rats fed mefloquine daily for 2 years. All surviving rats given 30 mg/kg/day had ocular lesions in both eyes characterized by retinal degeneration, opacity of the lens, and retinal edema. Similar but less severe lesions were observed in 80% of female and 22% of male rats fed 12.5 mg/kg/day for 2 years. At doses of 5 mg/kg/day, only corneal lesions were observed. They occurred in 9% of rats studied.
Male Wistar rats orally administered-mefloquine daily for 22 days at the equivalent human therapeutic plasma concentration showed CNS penetration of mefloquine, with a 30 to 50 fold greater brain/plasma drug ratio up to 10 days after the final dose administered.
6REFERENCES
1. Baudry S., Pham YT., Baune B., Vidrequin S., Crevoisier CH., Gimenez F., Fainotti R. (1997). Stereoselective passage of mefloquine through the blood brain barrier in the rat.
Manufactured In Canada By:
Manufactured For:
Rev. E 3/2019
7Medication Guide
Mefloquine Hydrochloride Tablets USP
Important:
Your doctor or pharmacist will give you an Information Wallet Card along with this
Medication Guide. It has important information about mefloquine and should be carried with
you at all times while you take mefloquine.
What is the most important information I should know about mefloquine?
Mefloquine can cause serious side effects, including:
1. Heart Problems.
Do not take halofantrine (used to treat malaria) or ketoconazole (used for fungal infections) with
mefloquine or within 15 weeks of your last dose of mefloquine. You may get serious heart
problems (problems with the electrical system of your heart called QT prolongation) that can lead
to death. Do not take quinine (Qualaquin) or quinidine (used to treat malaria or irregular heart
beat) with mefloquine. You may get serious heart problems.
2. Mental problems. Symptoms of serious mental problems may include:
- severe anxiety
- paranoia (feelings of mistrust towards others)
- hallucinations (seeing or hearing things that are not there)
- depression
- feeling restless
- unusual behavior
- feeling confused
Some people who take mefloquine think about suicide (putting an end to their life). Some people who were taking mefloquine committed suicide. It is not known if mefloquine was responsible for those suicides.
If you have any of these serious mental problems, or you develop other serious side effects or mental problems, you should contact your doctor right away as it may be necessary to stop taking mefloquine and use a different medicine to prevent malaria.
3. Problems with your body’s nervous system. Symptoms of serious nervous system
problems may include:
- dizziness
- a feeling that you or things around you are moving or spinning (vertigo)
- loss of balance
- ringing sound in your ears (tinnitus)
- convulsions (seizures) in people who already have seizures (epilepsy)
- convulsions (seizures) in people who take quinine or chloroquine (used to treat malaria) with mefloquine. Do not take quinine (Qualaquin) or chloroquine (Aralen) with mefloquine.
- unable to sleep (insomnia)
Dizziness, vertigo, tinnitus, and loss of balance can go on for months or years after
mefloquine is stopped or may become permanent in some people.
Important:
You need to take malaria prevention medicine before you travel to a malaria area, while
you are in a malaria area, and after you return from a malaria area.
- If you are told by a doctor to stop taking mefloquine because of the side effects or for other reasons, you will need to take different malaria medicine.
- If you do not have access to a doctor or to another medicine and have to stop taking mefloquine, leave the malaria area and contact a doctor as soon as possible because leaving the malaria area may not protect you from getting malaria. You will still need to take malaria prevention medicine for another 4 weeks after you leave the malaria area.
What is mefloquine?
Mefloquine is a prescription medicine used to prevent and treat malaria. Malaria can be a life-threatening infection. Mefloquine does not work for all types of malaria.
It is not known if mefloquine is safe and effective in children under 6 months old for the treatment of malaria. It is not known how well mefloquine works to prevent malaria in children
weighing less than 44 pounds (20 kilograms).
Who should not take mefloquine?
Do not take mefloquine if you have:
- depression or had depression recently
- had recent mental problems, including anxiety disorder, schizophrenia, or psychosis (losing touch with reality)
- seizures or had seizures (epilepsy or convulsions)
- an allergy to quinine, quinidine, mefloquine or any ingredients in mefloquine. See the end of this Medication Guide for a complete list of ingredients in mefloquine.
Talk to your doctor before you take mefloquine if you have any of the medical conditions listed above.
What should I tell my doctor before taking mefloquine?
Before taking mefloquine, tell your doctor about all your medical conditions, including if you have:
- heart disease
- liver problems
- seizures or epilepsy
- diabetes
- blood clotting problems or take blood thinner medicines (anticoagulants)
- mental problems
- are pregnant or plan to become pregnant. It is not known if mefloquine will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- You should use birth control while you take mefloquine and for 3 months after you stop mefloquine. If you have an unplanned pregnancy, talk to your doctor right away.
- are breastfeeding or plan to breastfeed. Mefloquine can pass into your breast milk and may harm your baby. Ask your doctor if you will need to stop breastfeeding or use a different medicine.
Contact your doctor right away if you have a fever after leaving a malaria area.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Mefloquine and other medicines may affect each other causing side effects.
How should I take mefloquine?
- Take mefloquine exactly as your doctor tells you to take it. Your doctor will tell you how many mefloquine tablets to take and when to take them.
- You will start taking mefloquine to prevent malaria between 1 to 3 weeks before you travel to a malaria area.
- Take mefloquine just after eating your largest meal of the day and with at least 1 cup (8 ounces) of water.
- Do not take mefloquine on an empty stomach.
- If you vomit after taking mefloquine, contact your doctor to see if you should take another dose.
- Continue taking mefloquine for 4 weeks after returning from a malaria area.
- Mefloquine tablets may be crushed and mixed with a small amount of water, milk or other beverage for children or other people unable to swallow mefloquine whole. Your doctor will tell you the correct dose for your child based on your child’s weight.
- If you take mefloquine for a year or longer, your doctor should check your:
- Use protective clothing, insect repellents, and bed nets to protect you from being bitten by mosquitoes. Medicine alone does not always stop you from catching malaria from mosquito bites.
What should I avoid while taking mefloquine?
Avoid activities such as driving a car or using heavy machinery or other activities needing alertness and careful movements (fine motor coordination) until you know how mefloquine affects you. You may feel dizzy or lose your balance. This could happen for months or years after you stop taking mefloquine and can be permanent in some cases. See “
What are the possible side effects of mefloquine?
See “
Mefloquine may cause serious side effects, including:
- liver problems
Call your healthcare provider right away if you have unexplained symptoms such as nausea or vomiting, stomach pain, fever, weakness, itching, unusual tiredness, loss of appetite, light colored bowel movements, dark colored urine, yellowing of your skin or the white of your eyes.
The most common side effects of mefloquine include:
- nausea
- vomiting
- diarrhea
- abdominal pain
- headache
The most common side effects in people who take mefloquine for treatment include:
- muscle pain
- fever
- chills
- skin rash
- fatigue
- loss of appetite
- irregular heart beat
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of mefloquine. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store mefloquine?
- Store mefloquine between 20ºC to 25ºC (68ºF to 77ºF)
- Safely throw away medicine that is out of date or no longer needed.
Keep mefloquine and all medicines out of the reach of children.
General information about the safe and effective use of mefloquine.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use mefloquine for a condition for which it was not prescribed. Do not give mefloquine to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about mefloquine. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about mefloquine that is written for health professionals.
What are the ingredients in mefloquine hydrochloride tablets?
Active ingredient: mefloquine hydrochloride
Inactive ingredients: colloidal silicon dioxide, corn starch, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch, poloxamer and talc.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured In Canada By:
Manufactured For:
Rev. D 3/2019
Manufactured In Canada By:
Manufactured For:
Rev. D 3/2019
8Mefloquine Hydrochloride
