Generic Name
Hydroxychloroquine
Brand Names
Plaquenil, Sovuna
FDA approval date: January 03, 2008
Classification: Antimalarial
Form: Tablet
What is Plaquenil (Hydroxychloroquine)?
Hydroxychloroquine sulfate tablets is an antimalarial and antirheumatic indicated for the: Treatment of uncomplicated malaria due to Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax in adult and pediatric patients.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Plaquenil (Hydroxychloroquine Sulfate)
1DOSAGE FORMS AND STRENGTHS
Tablets: 200 mg of hydroxychloroquine sulfate, white to off-white, film-coated tablet imprinted with “PLAQUENIL” on one face in black ink.
2CONTRAINDICATIONS
PLAQUENIL is contraindicated in patients with known hypersensitivity to 4-aminoquinoline compounds.
3ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
• Renal Toxicity
The following adverse reactions have been identified during post-approval use of 4-aminoquinoline drugs, including PLAQUENIL. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
4OVERDOSAGE
PLAQUENIL overdosage symptoms have an onset within 1–3 hours of ingestion. The following have been reported with PLAQUENIL overdosage:
5DESCRIPTION
PLAQUENIL (hydroxychloroquine sulfate) is an antimalarial and antirheumatic drug, chemically described as 2-[[4-[(7-Chloro-4- quinolyl) amino]pentyl] ethylamino]ethanol sulfate (1:1) with the molecular formula C
Hydroxychloroquine sulfate is a white or off-white crystalline powder, freely soluble in water; practically soluble in alcohol, chloroform, and ether.
6REFERENCES
1 Center for Disease Control and Prevention. Malaria.
https://www.cdc.gov/parasites/malaria/index.html
https://www.cdc.gov/parasites/malaria/index.html
7PATIENT COUNSELING INFORMATION
Important Administration Instructions
Advise the patient to take PLAQUENIL with food or milk and not to crush or divide the tablet.
Cardiomyopathy and Ventricular Arrhythmias
Inform the patient that serious cardiac effects, life-threatening and fatal cases have been reported with use of PLAQUENIL. Advise patients to seek medical attention immediately if they experience any symptoms of heart rhythm changes including fast or irregular heartbeat, lightheadedness, dizziness, or syncope [see .
Retinal Toxicity
Inform the patient that irreversible retinal damage has been observed in some patients with the use of PLAQUENIL. Advise patients of the importance of the ophthalmology visits for monitoring their eyes. Instruct patients to seek medical attention promptly if they experience decreased vision or decreased dark adaptation [see .
Serious Skin Reactions
Inform the patient that severe, life-threatening skin reactions have been reported with the use of PLAQUENIL. Advise the patient to seek medical attention immediately if experiencing any of the following signs and symptoms: blisters on the skin, eyes, lips or in the mouth, itching or burning, with or without fever [see .
Hepatotoxicity Associated with Porphyria Cutanea Tarda
Inform the patient that liver toxicity has been reported in when PLAQUENIL was used in patients with porphyria cutanea tarda. In some cases, PCT was diagnosed only after the occurrence of liver injury, when PLAQUENIL was prescribed for an approved indication. Advise the patient to seek medical attention if experiencing fatigue, rash, nausea, dark urine, or jaundice [see .
Skeletal Muscle Myopathy or Neuropathy
Inform the patient that muscle weakness and atrophy has been reported with PLAQUENIL use. Advise patients to report to the physician symptoms of muscle weakness [see .
Neuropsychiatric Reactions Including Suicidality
Alert patients to seek medical attention immediately if they experience new or worsening depression, suicidal thoughts, or other mood changes [see .
Hypoglycemia
Inform the patient that PLAQUENIL has been associated with severe hypoglycemia. Advise the patient to monitor blood sugar levels if possible and to seek medical attention if experiencing any of the signs and symptoms of hypoglycemia such as sweating, shakiness, weakness, dizziness, tachycardia, nausea, blurred vision, confusion, fainting, or loss of consciousness [see .
Pregnancy
Inform the patient that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to PLAQUENIL during pregnancy. Encourage patients to register by contacting 1-877-311-8972 [see Use in .
Manufactured for:
Advise the patient to take PLAQUENIL with food or milk and not to crush or divide the tablet.
Cardiomyopathy and Ventricular Arrhythmias
Inform the patient that serious cardiac effects, life-threatening and fatal cases have been reported with use of PLAQUENIL. Advise patients to seek medical attention immediately if they experience any symptoms of heart rhythm changes including fast or irregular heartbeat, lightheadedness, dizziness, or syncope [see .
Retinal Toxicity
Inform the patient that irreversible retinal damage has been observed in some patients with the use of PLAQUENIL. Advise patients of the importance of the ophthalmology visits for monitoring their eyes. Instruct patients to seek medical attention promptly if they experience decreased vision or decreased dark adaptation [see .
Serious Skin Reactions
Inform the patient that severe, life-threatening skin reactions have been reported with the use of PLAQUENIL. Advise the patient to seek medical attention immediately if experiencing any of the following signs and symptoms: blisters on the skin, eyes, lips or in the mouth, itching or burning, with or without fever [see .
Hepatotoxicity Associated with Porphyria Cutanea Tarda
Inform the patient that liver toxicity has been reported in when PLAQUENIL was used in patients with porphyria cutanea tarda. In some cases, PCT was diagnosed only after the occurrence of liver injury, when PLAQUENIL was prescribed for an approved indication. Advise the patient to seek medical attention if experiencing fatigue, rash, nausea, dark urine, or jaundice [see .
Skeletal Muscle Myopathy or Neuropathy
Inform the patient that muscle weakness and atrophy has been reported with PLAQUENIL use. Advise patients to report to the physician symptoms of muscle weakness [see .
Neuropsychiatric Reactions Including Suicidality
Alert patients to seek medical attention immediately if they experience new or worsening depression, suicidal thoughts, or other mood changes [see .
Hypoglycemia
Inform the patient that PLAQUENIL has been associated with severe hypoglycemia. Advise the patient to monitor blood sugar levels if possible and to seek medical attention if experiencing any of the signs and symptoms of hypoglycemia such as sweating, shakiness, weakness, dizziness, tachycardia, nausea, blurred vision, confusion, fainting, or loss of consciousness [see .
Pregnancy
Inform the patient that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to PLAQUENIL during pregnancy. Encourage patients to register by contacting 1-877-311-8972 [see Use in .
Manufactured for:
Advanz Pharma (US) Corp.
PLAQUENIL
8PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 100 TABLET BOTTLE
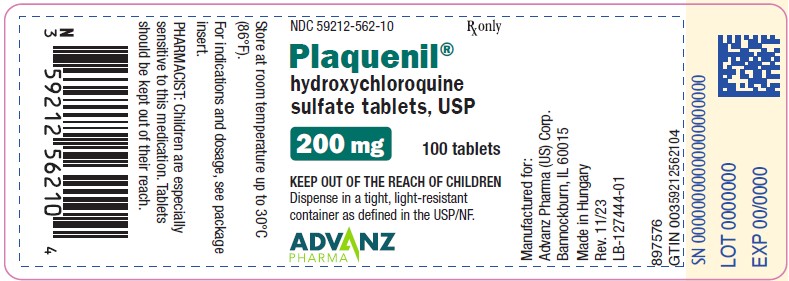
NDC 59212-562-10 Rx only
9PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 60 TABLET BOTTLE
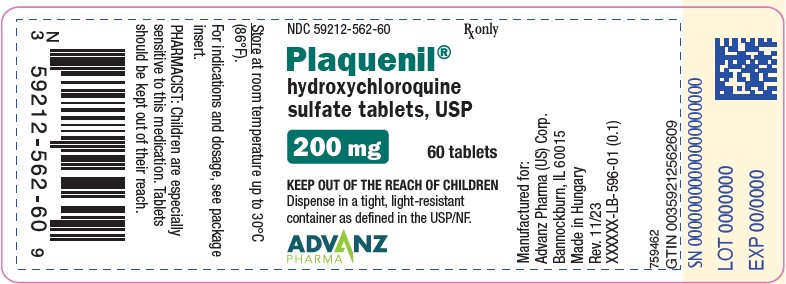
NDC 59212-562-60 Rx only