Brand Name
AdreView
Generic Name
Iobenguane I-123
View Brand Information FDA approval date: September 19, 2008
Form: Injection
What is AdreView (Iobenguane I-123)?
AdreView is a radiopharmaceutical agent for gamma-scintigraphy indicated for: use in the detection of primary or metastatic pheochromocytoma or neuroblastoma as an adjunct to other diagnostic tests.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Phase 3 Study of 131I-Metaiodobenzylguanidine (131I-MIBG) or ALK Inhibitor Therapy Added to Intensive Therapy for Children With Newly Diagnosed High-Risk Neuroblastoma (NBL)
Summary: This phase III trial studies iobenguane I-131 or lorlatinib and standard therapy in treating younger patients with newly-diagnosed high-risk neuroblastoma or ganglioneuroblastoma. Radioactive drugs, such as iobenguane I-131, may carry radiation directly to tumor cells and not harm normal cells. Lorlatinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Gi...
Related Latest Advances
Brand Information
AdreView (Iobenguane I-123)
1DOSAGE FORMS AND STRENGTHS
Single use vials containing 5 mL solution for intravenous injection (2 mCi/mL at calibration time).
2CONTRAINDICATIONS
AdreView is contraindicated in patients with known hypersensitivity to iobenguane or iobenguane sulfate.
3DRUG INTERACTIONS
The following drugs have the potential to decrease the uptake of norepinephrine and cause false negative imaging results: antihypertensives that deplete norepinephrine stores or inhibit reuptake (e.g., reserpine, labetalol), antidepressants that inhibit norepinephrine transporter function (e.g., amitriptyline and derivatives, imipramine and derivatives, selective serotonin reuptake inhibitors), sympathomimetic amines (e.g., phenylephrine, phenylpropanolamine, pseudoephedrine and ephedrine), and cocaine. Clinical studies have not determined which specific drugs may cause false negative imaging results nor whether all drugs in any specific pharmacologic class have the same potential to produce the negative imaging results. Increasing the dose of AdreView will not overcome any potential uptake limiting effect of these drugs. Before AdreView administration, discontinue (for at least 5 biological half-lives) drugs known or expected to reduce norepinephrine uptake, as clinically tolerated.
4OVERDOSAGE
The major manifestations of overdose relate predominantly to increased radiation exposure, with the long term risks for neoplasia.
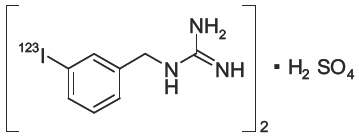
5DESCRIPTION
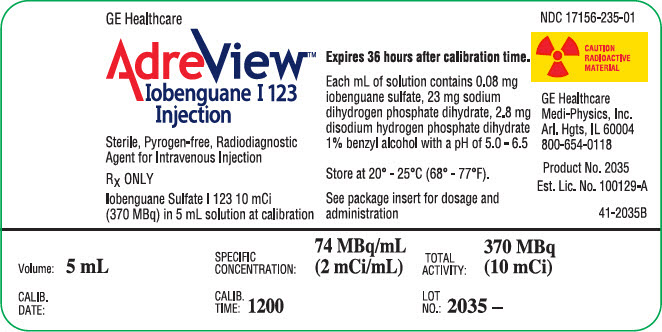
AdreView (Iobenguane I 123 Injection) is a sterile, pyrogen-free radiopharmaceutical for intravenous injection. Each mL contains 0.08 mg iobenguane sulfate, 74 MBq (2 mCi) of I 123 (as iobenguane sulfate I 123) at calibration date and time on the label, 23 mg sodium dihydrogen phosphate dihydrate, 2.8 mg disodium hydrogen phosphate dihydrate and 10.3 mg (1% v/v) benzyl alcohol with a pH of 5.0 – 6.5. Iobenguane sulfate I 123 is also known as I 123
5.1Physical Characteristics
Iodine 123 is a cyclotron-produced radionuclide that decays to Te 123 by electron capture and has a physical half-life of 13.2 hours.
5.2External Radiation
The specific gamma ray constant for iodine 123 is 1.6 R/mCi-hr at 1 cm. The first half value thickness of lead (Pb) for I 123 is 0.04 cm. The relative transmission of radiation emitted by the radionuclide that results from interposition of various thicknesses of Pb is shown in Table 4 (e.g., the use of 2.16 cm Pb will decrease the external radiation exposure by a factor of about 1,000).
6HOW SUPPLIED/STORAGE AND HANDLING
AdreView is supplied in 10 mL glass vials containing a total volume of 5 mL of solution with a total radioactivity of 370 MBq (10 mCi) at calibration time. Each vial is enclosed in a lead container of appropriate thickness.
NDC 17156-235-01
7PATIENT COUNSELING INFORMATION
Instruct patients to inform their physician or healthcare provider if they:
- are pregnant. Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with AdreView
- are breast feeding. Advise a lactating woman to interrupt breastfeeding and pump and discard breastmilk for at least 6 days (>10 physical half-lives) after AdreView administration in order to minimize radiation exposure to a breastfed infant
- are sensitive to iodine, an iodine-containing contrast agent or other products that contain iodine.
- are sensitive to Potassium Iodide Oral Solution, or Lugol's Solution.
- have reduced renal function.
Instruct patients to increase their level of hydration prior to receiving AdreView and to void frequently for the first 48 hours following AdreView administration.
8PRINCIPAL DISPLAY PANEL - 5 mL Vial Label
GE Healthcare
AdreView™
Sterile, Pyrogen-free, Radiodiagnostic
R
Iobenguane Sulfate I 123 10 mCi