Pembrolizumab
What is Keytruda (Pembrolizumab)?
Approved To Treat
Related Clinical Trials
Summary: This Phase 1, multicenter, open-label trial will assess the safety and feasibility of IFx-Hu2.0 as adjunctive therapy to pembrolizumab in adult patients (≥18 years) with non-cutaneous Merkel Cell Carcinoma. Nine subjects will receive IFx-Hu2.0 as a visceral lesion injection in a single lesion followed by pembrolizumab.
Summary: This is a global, multicenter, randomized, open-label, Phase 2/3 study of Dato-DXd plus carboplatin or cisplatin versus gemcitabine plus carboplatin or cisplatin in participants with la/mUC who progressed during or after EV plus pembrolizumab combination treatment. This trial will start with part A, Phase 2. During part A, Phase 2, preliminary efficacy and safety will be assessed, and the recommen...
Summary: This phase II/III trial compares the addition of radiation therapy to the usual treatment (immunotherapy with or without chemotherapy) versus (vs.) usual treatment alone in treating patients with non-small cell lung cancer that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced) or that has spread from where it first started (primary s...
Related Latest Advances
Brand Information
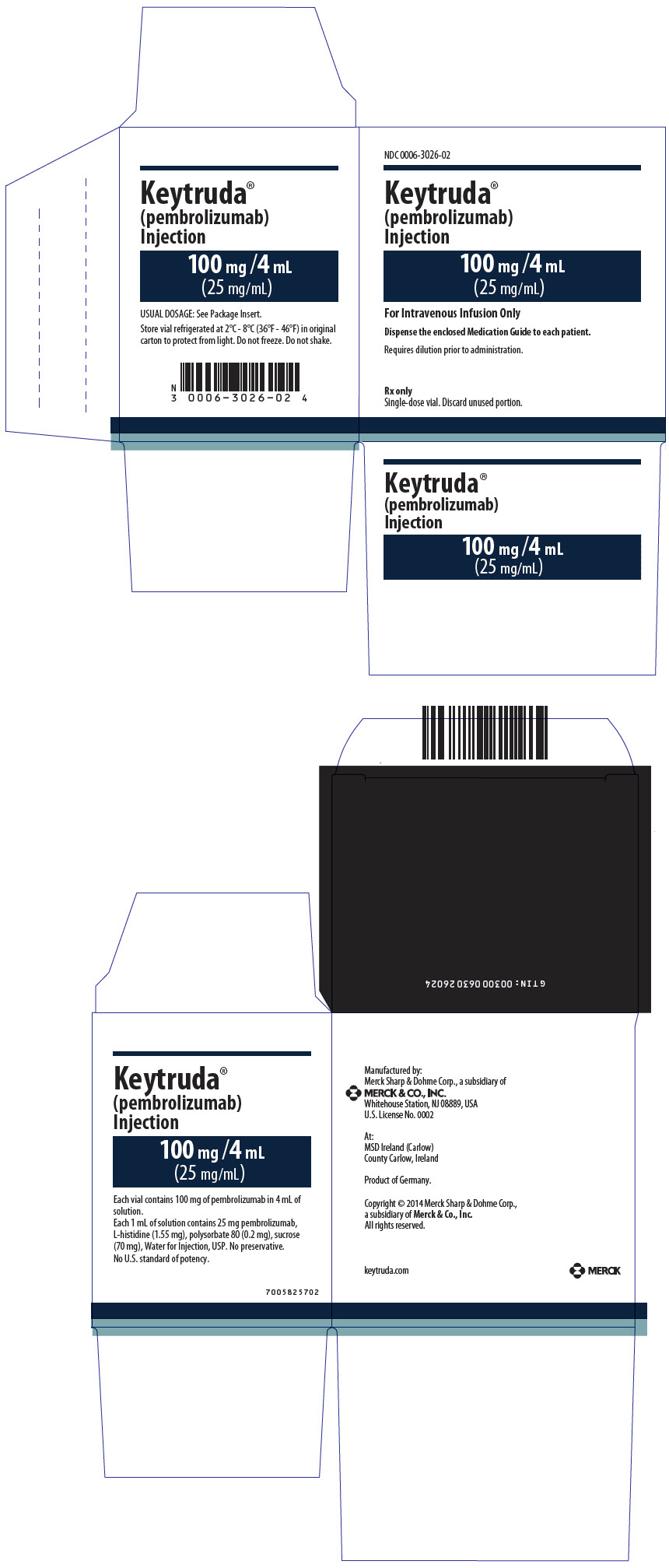
- Injection: 100 mg/4 mL (25 mg/mL) clear to slightly opalescent, colorless to slightly yellow solution in a single-dose vial
- Severe and fatal immune-mediated adverse reactions
- Infusion-related reactions
(pembrolizumab)
(25 mg/mL)
Single-dose vial. Discard unused portion.