Palynziq
What is Palynziq (Pegvaliase-Pqpz)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to determine if rapid drug desensitization (RDD) to Palynziq will improve drug tolerability and treatment persistence in adult patients on commercial Palynziq experiencing hypersensitivity reactions (HSRs) leading to treatment interruption or reduction of dose or dosing frequency. See Section 10.8 for full list of HSR preferred terms. Study details include: * Study dur...
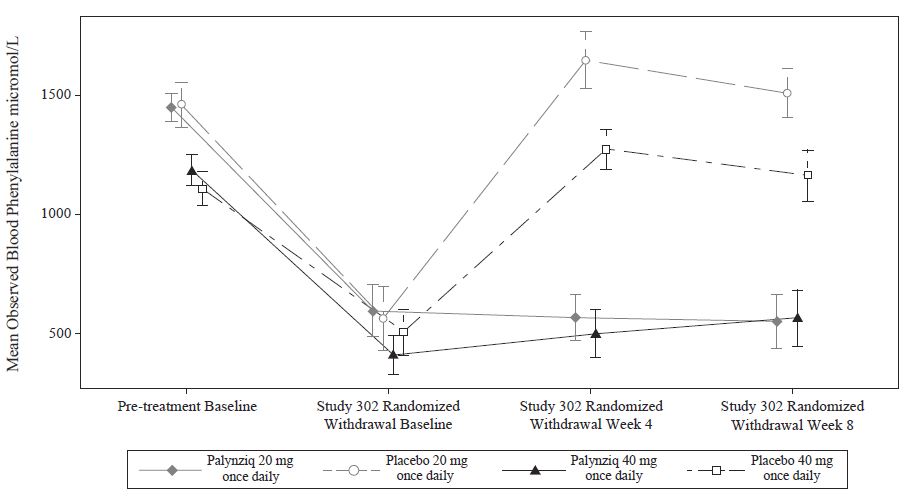
Summary: Phenylketonuria (PKU) is an inherited metabolic disorder that impairs the metabolism of the essential amino acid phenylalanine (Phe). Without stringent dietary control, Phe accumulates in the blood and brain of PKU patients, leading to severe cognitive deficits. Achieving metabolic control, defined as blood Phe levels within the range of 120-360 μmol/L, has been a significant challenge for PKU pat...
Summary: This is a 10-year multi-center, prospective, longitudinal, single arm study evaluating immunologic, inflammatory and laboratory parameters associated with long-term Palynziq treatment in subjects with phenylketonuria (PKU) in the United States (US). Subjects in the US for whom a clinical decision has been made that they will receive pegvaliase to treat their PKU within 30 days following the date o...
Related Latest Advances
Brand Information
Anaphylaxis has been reported after administration of Palynziq and may occur at any time during treatment Administer the initial dose of Palynziq under the supervision of a healthcare provider equipped to manage anaphylaxis, and closely observe patients for at least 60 minutes following injection. Prior to self‑injection, confirm patient competency with self‑administration, and patient’s and observer’s (if applicable) ability to recognize signs and symptoms of anaphylaxis and administer auto‑injectable epinephrine, if needed Consider having an adult observer for patients who may need assistance in recognizing and managing anaphylaxis during Palynziq treatment. If an adult observer is needed, the observer should be present during and for at least 60 minutes after Palynziq administration, should be able to administer auto‑injectable epinephrine, and call for emergency medical support upon its use Prescribe auto‑injectable epinephrine to all patients treated with Palynziq. Prior to the first dose, instruct the patient and observer (if applicable) how to recognize the signs and symptoms of anaphylaxis, how to properly administer auto‑injectable epinephrine, and to seek immediate medical care upon its use. Instruct patients to carry auto‑injectable epinephrine with them at all times during treatment with Palynziq Consider the risks and benefits of readministering Palynziq following an episode of anaphylaxis. If the decision is made to readminister Palynziq, readminister the first dose under the supervision of a healthcare provider equipped to manage anaphylaxis and closely observe the patient for at least 60 minutes following the dose Because of the risk of anaphylaxis, Palynziq is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Palynziq REMS
- Injection: 2.5 mg/0.5 mL single-dose prefilled syringe
- Injection: 10 mg/0.5 mL single-dose prefilled syringe
- Injection: 20 mg/mL single-dose prefilled syringe
- Anaphylaxis
- Other Hypersensitivity Reactions
- Store in refrigerator at 36°F to 46°F (2°C to 8°C) in its original carton to protect from light.
- Do not freeze or shake.
- For patients: If needed, store Palynziq in the original carton at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days. Record the date removed from refrigeration on the carton. Once stored at room temperature, do not return the product to the refrigerator.
- The shelf-life expires after storage at room temperature for 30 days, or after the expiration date on the product carton, whichever is earlier.
- Advise patients that Palynziq may cause hypersensitivity reactions, including anaphylaxis that can occur at any time. Instruct patients to recognize the signs and symptoms of anaphylaxis
- Instruct patients to carry auto-injectable epinephrine with them at all times during Palynziq treatment. Instruct the patient and observer (if applicable) on the appropriate use of auto‑injectable epinephrine for anaphylaxis
- Instruct patients who experience anaphylaxis to seek immediate medical care, discontinue therapy, and resume treatment only at the instruction of a healthcare provider
- Patients must be enrolled in the Palynziq REMS.
- Patients must be educated about the risk of anaphylaxis by a certified prescriber to ensure they understand the risks and benefits of treatment with Palynziq.
- Patients must fill a prescription for auto-injectable epinephrine and carry it with them at all times.
- Patients will be given a Palynziq Patient Wallet Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient and observer (if applicable) to immediately seek medical care. Advise the patient to show the Palynziq Wallet Card to other treating healthcare providers.
- Advise patients to monitor their dietary protein and phenylalanine intake throughout treatment with Palynziq, and adjust intake as directed by their healthcare provider based on blood phenylalanine concentrations
- Provide appropriate instruction for methods of self-injection, including careful review of the Palynziq Medication Guide and Instructions for Use. Instruct patients in the use of aseptic technique when administering Palynziq
- Inform patients that a healthcare provider will show them or their caregiver how to prepare to inject Palynziq before self-administering.
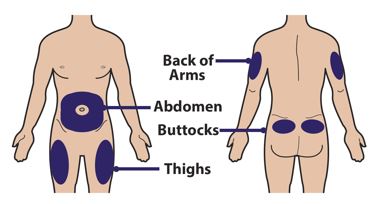
- Advise patients not to inject into moles, scars, birthmarks, bruises, rashes, or areas where the skin is hard, tender, red, damaged, burned, inflamed, or tattooed.
- Advise patients to rotate areas of injection with each dose.
- Advise patients that injection site infections may occur and to check the injection site for redness, swelling, or tenderness prior to injection. Instruct patients to contact their healthcare provider if signs or symptoms of an infection develop, persist, or worsen
- Advise patients to not administer Palynziq into the affected area until the infection has resolved.
- Advise patients to follow sharps disposal recommendations
- Advise patients that the shelf‑life expires after storage at room temperature for 30 days or after the expiration date on the product carton, whichever is earlier.
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
- Advise women who are exposed to Palynziq during pregnancy or who become pregnant within one month following the last dose of Palynziq that there is a pregnancy surveillance program that monitors pregnancy outcomes. Encourage these patients to report their pregnancy to BioMarin (1‑866‑906‑6100)
Palynziq prefilled syringe(s) in sealed tray(s). 1 gauze pad or cotton ball 1 alcohol pad 1 bandage 1 puncture resistant or sharps disposal container. See
Inject only 1 time with each Palynziq prefilled syringe. Do not pull back on the plunger at any time. Do not remove the needle cap until you are ready to inject.
Palynziq prefilled syringes come in 3 different strengths (See Figure C). Before you inject Palynziq, check each carton and syringe to make sure you have the right prefilled syringe for your prescribed dose.
- If the expiration date has passed,
- Do not warm up the prefilled syringe in any other way other than letting it sit at room temperature. Do not warm in a microwave and do not place in hot water.
- Throw away the prefilled syringe if it looks damaged or used, and use a new prefilled syringe for your injection. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
- Do not remove the needle cap from the prefilled syringe until Step 12.
- Do not shake or roll the syringe in your hands.
- It is normal to see air bubbles.
- Front middle of the thighs.
- The abdomen except for the 2 inch (5 centimeter) area directly around the belly button (navel).
- Do not inject into moles, scars, birthmarks, bruises, rashes, or areas where the skin is hard, tender, red, damaged, burned, inflamed, or tattooed.
- If you need more than 1 injection for your dose, the injection sites should be at least 2 inches away from each other. The second injection site can be on the same part of the body or a different part of the body (See Figures I and J).
- For each injection, change (rotate) your injection sites. Choose an injection site that is at least 2 inches away from the injection site that you used for the last injection. It can be on the same part of the body or a different part of the body (See Figures I and J).
- Do not touch the cleaned injection site.
- Do not remove the needle cap until you are ready to inject Palynziq.
- Do not use the prefilled syringe if it is dropped. Throw away the prefilled syringe if it is dropped and use a new prefilled syringe for your injection. See “Dispose of the used prefilled syringes” at the end of this Instructions for Use. Call BioMarin at 1‑866‑906‑6100 or your healthcare provider for help with Palynziq.
- Do not twist the needle cap during removal.
- Do not hold the prefilled syringe by the plunger or plunger head while taking the needle cap off.
- Before injecting Palynziq, check to make sure that the needle is not damaged or bent. Throw away the prefilled syringe if the needle is damaged or bent and use a new prefilled syringe for your injection. See
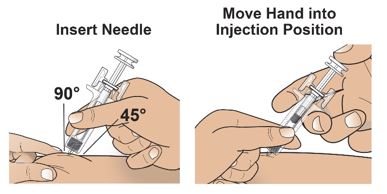
- Do not touch the plunger head while inserting the needle into the skin.
- Note: Do not give the injection in the same spot. The injection sites should be at least 2 inches away from each other. See Step 8 for information on choosing an injection site.
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- Store Palynziq in the refrigerator at 36°F to 46°F (2°C to 8°C) (See Figure T).
- If needed, you may store Palynziq at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days.
- Write the date that you remove Palynziq from the refrigerator on the carton.
- If stored at room temperature, do not put Palynziq back in the refrigerator.
- Keep Palynziq in the original carton to protect from light.
- Do not freeze or shake Palynziq.
- Throw away Palynziq if it has been kept at room temperature for 30 days and has not been used, or after the expiration date on the carton, whichever comes first.



