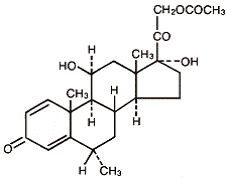
MethylPREDNISolone Acetate
What is Depo-Medrol (MethylPREDNISolone Acetate)?
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
Summary: Rotator cuff injuries are a major cause of severe pain, often significantly impacting patients\' sleep quality. For patients waiting for surgery or those not eligible for operative treatment, conservative treatment is recommended. In cases of minor injuries, physiotherapy is as effective as surgical intervention. The aim of this study is: (1) to compare the speed, effectiveness, and durability...
Summary: The goal of this study is to determine the effects of a corticosteroid administered to the psoas muscle following a transpsoas lateral lumbar interbody fusion (LLIF) on postoperative hip flexor weakness and thigh pain and numbness.
Summary: The objective of this phase 2 study is to investigate the efficacy of Dexamethasone sodium phosphate (DEX) plus Methylprednisolone acetate (MPA) in combination with plain bupivacaine (B) compared with Liposomal Bupivacaine (LB) in combination with plain bupivacaine on post-surgical pain control among patients undergoing unilateral total knee arthroplasty (TKA) to asses if perineural B-DEX-MPA will...
Related Latest Advances
Brand Information
NDC 0009-0274-01
(methylPREDNISolone acetate
injectable suspension, USP)
Rx only

Multidose Vial
(methylPREDNISolone
acetate injectable
suspension, USP)
as a Preservative

NDC 0009-0280-02
(methylPREDNISolone acetate
injectable suspension, USP)
(40 mg/mL)

(methylPREDNISolone acetate injectable suspension, USP)
(40 mg/mL)

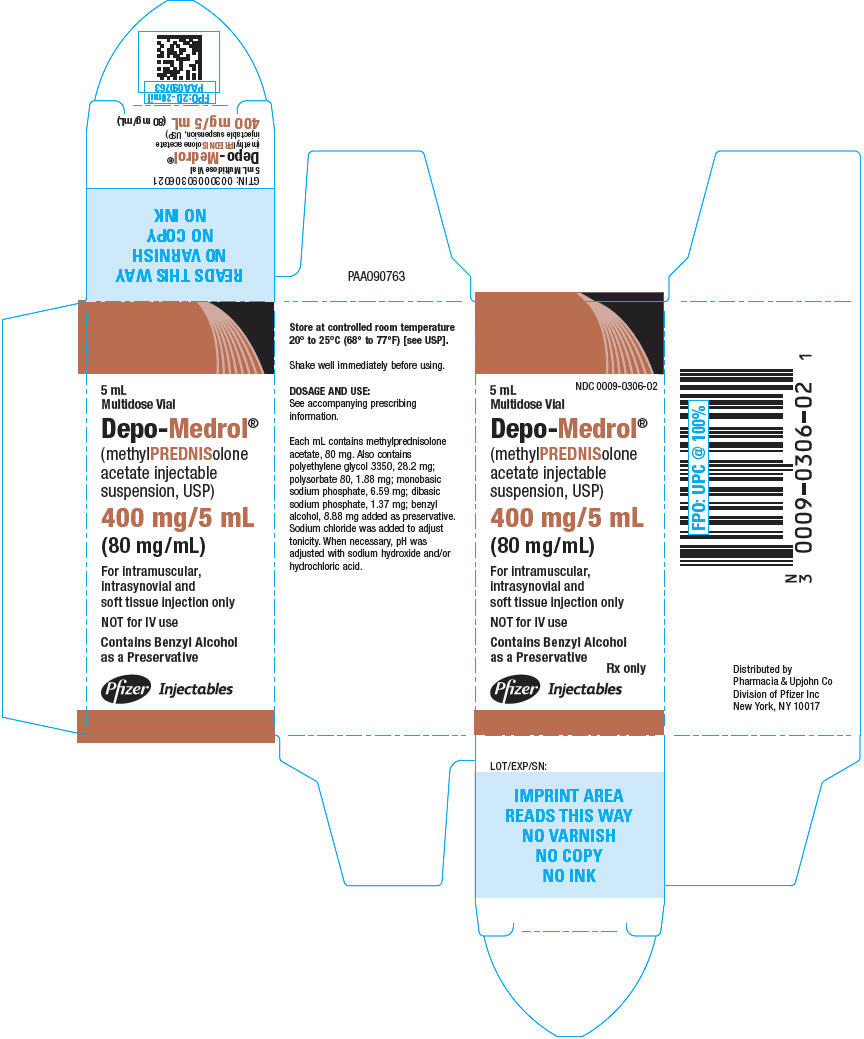
NDC 0009-0306-02
(methylPREDNISolone acetate
injectable suspension, USP)
(80 mg/mL)

Multidose Vial
(methylPREDNISolone
acetate injectable
suspension, USP)
(80 mg/mL)
as a Preservative