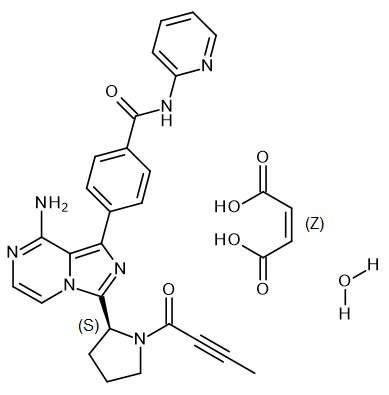
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the Warnings and Precautions reflect exposure to CALQUENCE 100 mg approximately every 12 hours in 1,764 patients with hematologic malignancies. Treatment includes CALQUENCE monotherapy in 1,256 patients in 9 trials, and CALQUENCE combinations in 508 patients in 3 trials. Among these recipients of CALQUENCE, 88% were exposed for at least 6 months and 80% were exposed for at least one year. In this pooled safety population, adverse reactions in ≥ 30% of 1,764 patients, excluding laboratory abnormalities, were diarrhea (37%), upper respiratory tract infection (36%), headache (35%), musculoskeletal pain (33%), lower respiratory tract infection (32%), and fatigue (32%). The most common grade 3 or 4 laboratory abnormalities (≥10%) were absolute neutrophil count decreased (31%), absolute lymphocyte count decreased (23%), platelets decreased (11%), and hemoglobin decreased (10%).
Previously Untreated Mantle Cell Lymphoma
The safety data described below reflect exposure to CALQUENCE (100 mg approximately every 12 hours, with or without BR) in patients with MCL
ECHO
The safety of CALQUENCE in combination with bendamustine and rituximab (CALQUENCE plus BR) was evaluated in 297 patients with previously untreated MCL in ECHO
The median duration of treatment with CALQUENCE was 28.6 months. A total of 171 (57.6%) patients were treated with CALQUENCE for ˃ 24 months and 122 (41.1%) patients were treated for ˃ 36 months.
Serious adverse reactions occurred in 69% of patients who received CALQUENCE plus BR. Serious adverse reactions reported in ≥ 2% of patients were pneumonia (23%; includes COVID-19 pneumonia), COVID-19 (20%; includes COVID-19 pneumonia), pyrexia (6%), second primary malignancy (7%), rash (3.4%), febrile neutropenia (3.4%), atrial fibrillation (3%), sepsis (2.7%), and anemia (2.4%). Fatal adverse reactions that occurred within 30 days of the last study treatment were reported in 12% who received CALQUENCE plus BR including COVID-19 (6%; includes COVID-19 pneumonia), pneumonia (1%), sepsis (0.3%), second primary malignancy (0.7%), and pneumonitis (0.3%).
Adverse reactions led to permanent discontinuation of CALQUENCE in 43%, dosage interruptions in 74%, and dosage reductions in 10% of patients. Adverse reactions that resulted in dosage modification in > 10% included infections, cytopenias, rashes, and gastrointestinal toxicity. Adverse reactions which resulted in permanent discontinuation of CALQUENCE in ≥ 4% of patients included COVID-19 (includes COVID-19 pneumonia) and neutropenia.
Table 4 and Table 5 summarize select adverse reactions and laboratory abnormalities observed in patients treated in ECHO.
Clinically relevant adverse reactions in < 15% of patients receiving CALQUENCE plus BR included bruising, abdominal pain, atrial fibrillation or flutter, and tumor lysis syndrome.
Grade 4 laboratory abnormalities in > 15% of patients treated with CALQUENCE plus BR include absolute lymphocyte count decreased (26%), absolute neutrophil count decreased (36%), and uric acid increased (17%).
Previously Treated Mantle Cell Lymphoma
ACE-LY-004
The safety data described in this section reflect exposure to CALQUENCE (100 mg approximately every 12 hours) in 124 patients with previously treated MCL in Trial LY-004
The most common adverse reactions (≥ 20%) of any grade were anemia, thrombocytopenia, headache, neutropenia, diarrhea, fatigue, myalgia, and bruising. Grade 1 severity for the non-hematologic, most common events were as follows: headache (25%), diarrhea (16%), fatigue (20%), myalgia (15%), and bruising (19%). The most common Grade ≥ 3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea.
Dose reductions and discontinuation due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively.
Tables 6 and 7 present the frequency category of adverse reactions observed in patients with MCL treated with CALQUENCE.
*Per NCI CTCAE version 4.03.
a Bruising: Includes all terms containing ‘bruise,’ ‘contusion,’ ‘petechiae,’ or ‘ecchymosis’.
b Rash: Includes all terms containing ‘rash’.
c Hemorrhage: Includes all terms containing ‘hemorrhage’ or ‘hematoma’.
*Per NCI CTCAE version 4.03; based on laboratory measurements and adverse reactions.
Increases in creatinine to 1.5 to 3 times the upper limit of normal (ULN) occurred in 4.8% of patients.
Chronic Lymphocytic Leukemia
The safety data described below reflect exposure to CALQUENCE (100 mg approximately every 12 hours, with or without obinutuzumab) in 511 patients with CLL from two randomized controlled clinical trials
The most common adverse reactions (≥ 30%) of any grade in patients with CLL were anemia, neutropenia, thrombocytopenia, headache, upper respiratory tract infection, and diarrhea.
ELEVATE-TN
The safety of CALQUENCE plus obinutuzumab (CALQUENCE+G), CALQUENCE monotherapy, and obinutuzumab plus chlorambucil (GClb) was evaluated in a randomized, multicenter, open-label, actively controlled trial in 526 patients with previously untreated CLL
Patients randomized to the CALQUENCE+G arm were treated with CALQUENCE and obinutuzumab in combination for six cycles, then with CALQUENCE as monotherapy until disease progression or unacceptable toxicity. Patients initiated obinutuzumab on Day 1 of Cycle 2, continuing for a total of 6 cycles. Patient randomized to CALQUENCE monotherapy received CALQUENCE approximately every 12 hours until disease progression or unacceptable toxicity. The trial required age ≥ 65 years of age or 18 to < 65 years of age with a total Cumulative Illness Rating Scale (CIRS) > 6 or creatinine clearance of 30 to 69 mL/min, hepatic transaminases ≤ 3 times ULN and total bilirubin ≤ 1.5 times ULN, and allowed patients to receive antithrombotic agents other than warfarin or equivalent vitamin K antagonists.
During randomized treatment, the median duration of exposure to CALQUENCE in the CALQUENCE+G and CALQUENCE monotherapy arms was 27.7 months (range 0.3 to 40 months), with 95% and 92% and 89% and 86% of patients with at least 6 months and 12 months of exposure, respectively. In the obinutuzumab and chlorambucil arm the median number of cycles was 6 with 84% of patients receiving at least 6 cycles of obinutuzumab, 70% of patients received at least 6 cycles of chlorambucil. Eighty-five percent of patients in the CALQUENCE+G arm received at least 6 cycles of obinutuzumab.
In the CALQUENCE+G and CALQUENCE monotherapy arms, fatal adverse reactions that occurred in the absence of disease progression and with onset within 30 days of the last study treatment were reported in 2% for each treatment arm, most often from infection. Serious adverse reactions were reported in 39% of patients in the CALQUENCE+G arm and 32% in the CALQUENCE monotherapy arm, most often due to events of pneumonia (2.8% to 7%).
In the CALQUENCE+G arm, adverse reactions led to treatment discontinuation in 11% of patients and a dose reduction of CALQUENCE in 7% of patients. In the CALQUENCE monotherapy arm, adverse reactions led to discontinuation in 10% and dose reduction in 4% of patients.
Tables 8 and 9 present adverse reactions and laboratory abnormalities identified in the ELEVATE-TN trial.
*Per NCI CTCAE version 4.03.
† Includes any adverse reactions involving infection or febrile neutropenia.
‡ Includes 3 fatal cases in the CALQUENCE plus obinutuzumab arm, 3 fatal cases in the CALQUENCE monotherapy arm and 1 fatal case in the obinutuzumab plus chlorambucil arm.
§ Includes upper respiratory tract infection, nasopharyngitis and sinusitis.
a Includes pneumonia, lower respiratory tract infection, bronchitis, bronchiolitis, tracheitis, and lung infection.
b Derived from adverse reaction and laboratory data.
c Includes neutropenia, neutrophil count decreased, and related laboratory data.
d Includes anemia, red blood cell count decreased, and related laboratory data.
e Includes thrombocytopenia, platelet count decreased, and related laboratory data.
f Includes lymphocytosis, lymphocyte count increased, and related laboratory data.
g Includes back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal discomfort, myalgia, neck pain, pain in extremity and spinal pain.
h Includes asthenia, fatigue, and lethargy.
i Includes bruise, contusion, and ecchymosis.
j Includes rash, dermatitis, and other related terms.
k Includes hemorrhage, hematoma, hemoptysis, hematuria, menorrhagia, hemarthrosis, and epistaxis.
Other clinically relevant adverse reactions (all grades incidence < 15%) in recipients of CALQUENCE (CALQUENCE in combination with obinutuzumab and monotherapy) included:
- Neoplasms: second primary malignancy (10%), non-melanoma skin cancer (5%)
- Cardiac disorders: atrial fibrillation or flutter (3.6%), hypertension (5%)
- Infection: herpesvirus infection (6%)
Increases in creatinine to 1.5 to 3 times ULN occurred in 3.9% and 2.8% of patients in the CALQUENCE combination arm and monotherapy arm, respectively.
ASCEND
The safety of CALQUENCE in patients with relapsed or refractory CLL was evaluated in a randomized, open-label study (ASCEND)
In ASCEND, 154 patients received CALQUENCE (100 mg approximately every 12 hours until disease progression or unacceptable toxicity), 118 received idelalisib (150 mg approximately every 12 hours until disease progression or unacceptable toxicity) with up to 8 infusions of a rituximab product, and 35 received up to 6 cycles of bendamustine and a rituximab product. The median age overall was 68 years (range: 32-90); 67% were male; 92% were white; and 88% had an ECOG performance status of 0 or 1.
In the CALQUENCE arm, serious adverse reactions occurred in 29% of patients. Serious adverse reactions in > 5% of patients who received CALQUENCE included lower respiratory tract infection (6%). Fatal adverse reactions within 30 days of the last dose of CALQUENCE occurred in 2.6% of patients, including from second primary malignancies and infection.
In recipients of CALQUENCE, permanent discontinuation due to an adverse reaction occurred in 10% of patients, most frequently due to second primary malignancies followed by infection. Adverse reactions led to dosage interruptions of CALQUENCE in 34% of patients, most often due to respiratory tract infections followed by neutropenia, and dose reduction in 3.9% of patients.
Selected adverse reactions are described in Table 10 and non-hematologic laboratory abnormalities are described in Table 11. These tables reflect exposure to CALQUENCE with median duration of 15.7 months with 94% of patients on treatment for greater than 6 months and 86% of patients on treatment for greater than 12 months. The median duration of exposure to idelalisib was 11.5 months with 72% of patients on treatment for greater than 6 months and 48% of patients on treatment for greater than 12 months. Eighty-three percent of patients completed 6 cycles of bendamustine and rituximab product.
* Per NCI CTCAE version 4.03.
† Includes any adverse reactions involving infection or febrile neutropenia.
‡ Includes 1 fatal case in the CALQUENCE monotherapy arm and 1 fatal case in the Idelalisib plus Rituximab arm.
§ Includes upper respiratory tract infection, rhinitis and nasopharyngitis.
a Includes pneumonia, lower respiratory tract infection, bronchitis, bronchiolitis, tracheitis, and lung infection.
b Derived from adverse reaction and laboratory data.
c Includes neutropenia, neutrophil count decreased, and related laboratory data.
d Includes anemia, red blood cell decreased, and related laboratory data.
e Includes thrombocytopenia, platelet count decreased, and related laboratory data.
f Includes lymphocytosis, lymphocyte count increased and related laboratory data.
g Includes colitis, diarrhea, and enterocolitis.
h Includes hemorrhage, hematoma, hemoptysis, hematuria, menorrhagia, hemarthrosis, and epistaxis.
i Includes asthenia, fatigue, and lethargy.
j Includes back pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal discomfort, pain in extremity, myalgia, spinal pain and bone pain.
Other clinically relevant adverse reactions (all grades incidence < 15%) in recipients of CALQUENCE included:
- Skin and subcutaneous disorders: bruising (10%), rash (9%)
- Neoplasms: second primary malignancy (12%), non-melanoma skin cancer (6%)
- Musculoskeletal and connective tissue disorders: arthralgia (8%)
- Cardiac disorders: atrial fibrillation or flutter (5%), hypertension (3.2%)
- Infection: herpesvirus infection (4.5%)
Increases in creatinine to 1.5 to 3 times ULN occurred in 1.3% of patients who received CALQUENCE.