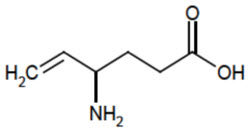
Vigabatrin
What is Vigadrone (Vigabatrin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of the study is to evaluate the efficacy, tolerability, and safety of vigabatrin versus rapamycin as a preventive treatment in infants with Tuberous Sclerosis Complex (TSC).
Summary: This is a phase II clinical trial in which children with refractory infantile spasms (also called epileptic spasms or West syndrome) will be treated with fenfluramine, to evaluate efficacy, safety, and tolerability. Patients with infantile spasms that have not responded to treatment with vigabatrin and ACTH we will be invited to participate. Study participants will undergo baseline video-EEG, rece...
Summary: Infantile spasms (IS) are seizures associated with a severe infantile epileptic encephalopathy. Both cessation of spasms and electrographic response are necessary for the best neurodevelopmental outcomes. Adrenocorticotrophic hormone (ACTH), or prednisolone, or vigabatrin are considered the first-line treatment individually. However, ACTH expense and availability are the barriers in developing cou...
Related Latest Advances
Brand Information
- VIGADRONE can cause permanent bilateral concentric visual field constriction, including tunnel vision that can result in disability. In some cases, VIGADRONE also can damage the central retina and may decrease visual acuity
- The onset of vision loss from VIGADRONE is unpredictable and can occur within weeks of starting treatment or sooner, or at any time after starting treatment, even after months or years.
- Symptoms of vision loss from VIGADRONE are unlikely to be recognized by patients or caregivers before vision loss is severe. Vision loss of milder severity, while often unrecognized by the patient or caregiver, can still adversely affect function.
- The risk of vision loss increases with increasing dose and cumulative exposure, but there is no dose or exposure known to be free of risk of vision loss.
- Vision assessment is recommended at baseline (no later than 4 weeks after starting VIGADRONE), at least every 3 months during therapy, and about 3 to 6 months after the discontinuation of therapy.
- Once detected, vision loss due to VIGADRONE is not reversible. It is expected that, even with frequent monitoring, some patients will develop severe vision loss.
- Consider drug discontinuation, balancing benefit and risk, if vision loss is documented.
- Risk of new or worsening vision loss continues as long as VIGADRONE is used. It is possible that vision loss can worsen despite discontinuation of VIGADRONE.
- Because of the risk of vision loss, VIGADRONE should be withdrawn from patients with refractory complex partial seizures who fail to show substantial clinical benefit within 3 months of initiation and within 2 to 4 weeks of initiation for patients with infantile spasms, or sooner if treatment failure becomes obvious. Patient response to and continued need for VIGADRONE should be periodically reassessed.
- VIGADRONE should not be used in patients with, or at high risk of, other types of irreversible vision loss unless the benefits of treatment clearly outweigh the risks.
- VIGADRONE should not be used with other drugs associated with serious adverse ophthalmic effects such as retinopathy or glaucoma unless the benefits clearly outweigh the risks.
- Use the lowest dosage and shortest exposure to VIGADRONE consistent with clinical objectives
- Permanent Vision Loss
- Magnetic Resonance Imaging (MRI) Abnormalities in Infants
- Neurotoxicity
- Suicidal Behavior and Ideation
- Withdrawal of Antiepileptic Drugs (AEDs)
- Anemia
- Somnolence and Fatigue
- Peripheral Neuropathy
- Weight Gain
- Edema