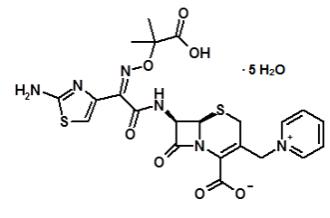
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Patients
AVYCAZ was evaluated in six active-controlled clinical trials in patients with cIAI, cUTI, including pyelonephritis, or HABP/VABP. These trials included two Phase 2 trials, one in cIAI and one in cUTI, as well as four Phase 3 trials, one in cIAI, one in cUTI (Trial 1), one in cIAI or cUTI due to ceftazidime non-susceptible pathogens (Trial 2), and one in HABP/VABP. Data from cUTI Trial 1 served as the primary dataset for AVYCAZ safety findings in cUTI as there was a single comparator. cUTI Trial 2 had an open-label design as well as multiple comparator regimens which prevented pooling but provided supportive information. The six clinical trials included a total of 1809 adult patients treated with AVYCAZ and 1809 patients treated with comparators.
Complicated Intra-abdominal Infections
The Phase 3 cIAI trial included 529 adult patients treated with AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes every 8 hours plus 0.5 grams metronidazole administered intravenously over 60 minutes every 8 hours and 529 patients treated with meropenem. The median age of patients treated with AVYCAZ was 50 years (range 18 to 90 years) and 22.5% of patients were 65 years of age or older. Patients were predominantly male (62%) and Caucasian (76.6%).
Treatment discontinuation due to an adverse reaction occurred in 2.6% (14/529) of patients receiving AVYCAZ plus metronidazole and 1.3% (7/529) of patients receiving meropenem.
Adverse reactions occurring at 5% or greater in patients receiving AVYCAZ plus metronidazole were diarrhea, nausea, and vomiting.
Table 11 lists adverse reactions occurring in 1% or more of patients receiving AVYCAZ plus metronidazole and with incidences greater than the comparator in the Phase 3 cIAI clinical trial.
Increased Mortality
In the Phase 3 cIAI trial, death occurred in 2.5% (13/529) of patients who received AVYCAZ plus metronidazole and in 1.5% (8/529) of patients who received meropenem. Among a subgroup of patients with baseline CrCl 30 to less than or equal to 50 mL/min, death occurred in 19.5% (8/41) of patients who received AVYCAZ plus metronidazole and in 7.0% (3/43) of patients who received meropenem. Within this subgroup, patients treated with AVYCAZ received a 33% lower daily dose than is currently recommended for patients with CrCl 30 to less than or equal to 50 mL/min
Complicated Urinary Tract Infections, Including Pyelonephritis
The Phase 3 cUTI Trial 1 included 511 adult patients treated with AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes every 8 hours and 509 patients treated with doripenem; in some patients parenteral therapy was followed by a switch to an oral antimicrobial agent
There were no deaths in Trial 1. Treatment discontinuation due to adverse reactions occurred in 1.4% (7/511) of patients receiving AVYCAZ and 1.2% (6/509) of patients receiving doripenem.
The most common adverse reactions occurring in 3% of cUTI patients treated with AVYCAZ were nausea and diarrhea.
Table 12 lists adverse reactions occurring in 1% or more of patients receiving AVYCAZ and with incidences greater than the comparator in Trial 1.
Hospital-acquired Bacterial Pneumonia/Ventilator-associated Bacterial Pneumonia
The Phase 3 HABP/VABP trial included 436 adult patients treated with AVYCAZ 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes and 434 patients treated with meropenem. The median age of patients treated with AVYCAZ was 66 years (range 18 to 89 years) and 54.1% of patients were 65 years of age or older. Patients were predominantly male (74.5%) and Asian (56.2%).
Death occurred in 9.6% (42/ 436) of patients who received AVYCAZ and in 8.3% (36/434) of patients who received meropenem. Treatment discontinuation due to an adverse reaction occurred in 3.7% (16/436) of patients receiving AVYCAZ and 3% (13/434) of patients receiving meropenem.
Adverse reactions occurring at 5% or greater in patients receiving AVYCAZ were diarrhea and vomiting.
Table 13 lists selected adverse reactions occurring in 1% or more of patients receiving AVYCAZ and with incidences greater than the comparator in the Phase 3 HABP/VABP clinical trial.
Other Adverse Reactions of AVYCAZ and Ceftazidime in Adults
Direct Coombs’ Test Seroconversion with AVYCAZ
In the Phase 3 trials, seroconversion from a negative to a positive direct Coombs’ test result among patients with an initial negative Coombs’ test and at least one follow up test occurred in 3% (cUTI), 12.9% (cIAI), and 21.4% (HABP/VABP) of patients receiving AVYCAZ and 0.9% (cUTI), 3% (cIAI) and 7% (HABP/VABP) of patients receiving a carbapenem comparator.
Less Common Adverse Reactions with AVYCAZ
The following selected adverse reactions were reported in AVYCAZ-treated patients at a rate of less than 1% in the Phase 3 trials and are not described elsewhere in the labeling.
Blood and lymphatic disorders– Thrombocytopenia, Thrombocytosis, Leukopenia
General disorders and administration site conditions–Injection site phlebitis
Infections and infestations –Candidiasis
Investigations– Increased aspartate aminotransferase, Increased alanine aminotransferase, Increased gamma-glutamyl transferase
Metabolism and nutrition disorders– Hypokalemia
Nervous system disorders –Dysgeusia
Renal and urinary disorders– Acute kidney injury, Renal impairment, Nephrolithiasis
Skin and subcutaneous tissue disorders– Rash, Rash maculo-papular, Urticaria
Psychiatric disorders –Anxiety
Adverse Reactions with Ceftazidime
Additionally, adverse reactions reported with ceftazidime alone that were not reported in AVYCAZ-treated patients in the Phase 3 trials are listed below:
Blood and lymphatic disorders –Agranulocytosis, Hemolytic anemia, Lymphocytosis, Neutropenia, Eosinophilia
General disorders and administration site conditions – Infusion site inflammation, Injection site hematoma, Injection site thrombosis
Hepatobiliary disorders –Jaundice
Investigations –Increased blood lactate dehydrogenase,Prolonged prothrombin time
Nervous system disorders –Paresthesia, seizures, encephalopathy, coma, asterixis, neuromuscular excitability, myoclonia
Renal and urinary disorders –Tubulointerstitial nephritis
Reproductive and breast disorders –Vaginal inflammation
Hypersensitivity Reactions–Anaphylaxis, Angioedema, Erythema multiforme, Stevens-Johnsonsyndrome, Toxic epidermal necrolysis
Clinical Trials Experience in Pediatric Patients
Pediatric Patients Aged 3 months to less than 18 years
AVYCAZ was evaluated in 128 pediatric patients aged 3 months to < 18 years in two single-blind, randomized, active-controlled clinical trials, one in patients with cUTI and the other in patients with cIAI. Safety data from the two studies were pooled. The AVYCAZ dosing regimen was the same in both of these trials
There were no deaths reported in the trials of cUTI, cIAI, and HABP/VABP in pediatric patients aged 3 months and older. Treatment discontinuation due to adverse reactions in the pediatric cUTI and cIAI trials occurred in 2.3% (3/128) of patients receiving AVYCAZ and 0/50 of patients receiving comparator drugs.
The most common adverse reactions occurring in greater than 3% of pediatric patients aged 3 months to < 18 years treated with AVYCAZ were vomiting, diarrhea, rash, and infusion site phlebitis.
Pediatric Patients less than 3 months of Age
AVYCAZ was also evaluated in a trial enrolling 46 pediatric patients less than three months of age as follows: infants > 28 days to < 3 months (N=17), term neonates from birth to 28 days, (N=13), pre-term neonates from birth (gestational age ≥ 31 weeks) to 28 days (N=16). The median age of patients treated with AVYCAZ was 24 days. In this single-arm trial, 25 patients with a suspected or confirmed bacterial infection received a single-dose of AVYCAZ and 21 patients with suspected or confirmed serious gram-negative infections received multiple doses of AVYCAZ
There was one death reported in the trial for pediatric patients less than 3 months of age. There were no treatment discontinuations due to adverse reactions. The most common adverse reactions occurring in greater than 3% of pediatric patients less than 3 months of age were vomiting and increased transaminases.
The safety profile of AVYCAZ in pediatric patients was similar to adults with cIAI, cUTI, and HABP/VABP treated with AVYCAZ.