Intensol
What is TaperDex 12-day (Intensol)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to learn about the study medicine called elranatamab.This study aims to compare elranatamab to other medicines for the treatment of MM (a type of cancer). This study is seeking participants who: * Are 18 years of age or older and have MM. * Have received treatments before for MM. * Have MM that has returned or not responded to their most recent treatment. Half of the p...
Summary: Elranatamab is a bispecific antibody: binding of elranatamab to CD3-expressing T-cells and BCMA-expressing multiple myeloma cells causes targeted T-cell-mediated cytotoxicity. The main purpose of the study is to evaluate if the combination of Elranatamab, Daratumumab and Lenalidomide or Elranatamab and Lenalidomide offers superior clinical benefit compared with the combination of Daratumumab, Lena...
Summary: The purpose of this clinical trial is to (1) learn whether the BCMA-CD3 bispecific antibody elranatamab can provide more benefit to people with multiple myeloma compared to a combination therapy including daratumumab, pomalidomide, and dexamethasone, and (2) learn about the safety and activity of elranatamab in combination with the anti-CD38 monoclonal antibody daratumumab. People with multiple my...
Related Latest Advances
Brand Information
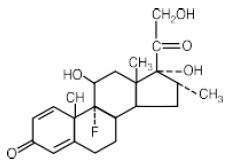
After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage that maintains an adequate clinical response is reached.
Situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment. In this latter situation it may be necessary to increase the dosage of the corticosteroid for a period of time consistent with the patient’s condition. If after long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
In the treatment of acute exacerbations of multiple sclerosis, daily doses of 30 mg of TaperDex 12-Day for a week followed by 4 to 12 mg every other day for one month have been shown to be effective (see PRECAUTIONS, Neuropsychiatric). In pediatric patients, the initial dose of TaperDex 12-Day may vary depending on the specific disease entity being treated. The range of initial doses is 0.02 to 0.3 mg/kg/day in three or four divided doses (0.6 to 9 mg/m2body surface area/day). For the purpose of comparison, the following is the equivalent milligram dosage of the various corticosteroids:
In acute, self-limited allergic disorders or acute exacerbations of chronic allergic disorders, the following dosage schedule combining parenteral and oral therapy is suggested: Dexamethasone Sodium Phosphate injection, USP 4 mg per mL:
1 or 2 mL, intramuscularly
Dexamethasone Tablets, USP, 1.5 mg, one-half tablet:
Second Day
2 tablets in two divided doses
Third Day
2 tablets in two divided doses
Fourth Day
1 tablet in two divided doses
Fifth Day
One half tablet
Sixth Day
One half tablet
Seventh Day
No treatment
Eighth Day
Follow-up visit

