Brand Name
Cerebyx
Generic Name
Fosphenytoin
View Brand Information FDA approval date: August 06, 2007
Classification: Anti-epileptic Agent
Form: Injection
What is Cerebyx (Fosphenytoin)?
CEREBYX is indicated for the treatment of generalized tonic-clonic status epilepticus and prevention and treatment of seizures occurring during neurosurgery. CEREBYX can also be substituted, short-term, for oral phenytoin. CEREBYX should be used only when oral phenytoin administration is not possible [see Dosage and Administration.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Platform Protocol to Investigate Post-Transplant Cyclophosphamide-Based Graft-Versus-Host Disease Prophylaxis in Patients With Hematologic Malignancies Undergoing Mismatched Unrelated Donor Peripheral Blood Stem Cell Transplantation
Summary: The purpose of this clinical trial is to compare drug combinations to learn which drugs work best to prevent graft-versus-host-disease (GVHD) in people who have received a stem cell transplant. The source of stem cells is from someone who is not related and has a different blood cell type than the study participant. The researchers will compare the new drug combination to a standard drug combinati...
Related Latest Advances
Brand Information
CEREBYX (Fosphenytoin Sodium)
WARNING: CARDIOVASCULAR RISK ASSOCIATED WITH RAPID INFUSION RATES
The rate of intravenous CEREBYX administration should not exceed 150 mg phenytoin sodium equivalents (PE) per minute in adults and 2 mg PE/kg/min (or 150 mg PE/min, whichever is slower) in pediatric patients because of the risk of severe hypotension and cardiac arrhythmias. Careful cardiac monitoring is needed during and after administering intravenous CEREBYX. Although the risk of cardiovascular toxicity increases with infusion rates above the recommended infusion rate, these events have also been reported at or below the recommended infusion rate. Reduction in rate of administration or discontinuation of dosing may be needed
1INDICATIONS AND USAGE
CEREBYX is indicated for the treatment of generalized tonic-clonic status epilepticus and prevention and treatment of seizures occurring during neurosurgery. CEREBYX can also be substituted, short-term, for oral phenytoin. CEREBYX should be used only when oral phenytoin administration is not possible
2DOSAGE FORMS AND STRENGTHS
CEREBYX Injection is a clear, colorless to pale yellow solution available as 50 mg phenytoin sodium equivalents (PE) per mL in:
- 10 mL single-dose injection vials, each containing 500 mg PE/10 mL (50 mg PE/mL)
- 2 mL single-dose injection vials, each containing 100 mg PE/2 mL (50 mg PE/mL)
3CONTRAINDICATIONS
CEREBYX is contraindicated in patients with:
- A history of hypersensitivity to CEREBYX or its inactive ingredients, or to phenytoin or other hydantoins
- Sinus bradycardia, sino-atrial block, second and third degree A-V block, or Adams-Stokes syndrome because of the effect of parenteral phenytoin or CEREBYX on ventricular automaticity.
- A history of prior acute hepatotoxicity attributable to CEREBYX or phenytoin
- Coadministration with delavirdine because of the potential for loss of virologic response and possible resistance to delavirdine or to the class of non-nucleoside reverse transcriptase inhibitors.
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Cardiovascular Risk Associated with Rapid Infusion
- Withdrawal Precipitated Seizure, Status Epilepticus
- Serious Dermatologic Reactions
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
- Hypersensitivity
- Angioedema
- Hepatic Injury
- Hematopoietic Complications
- Sensory Disturbances
- Local Toxicity (Including Purple Glove Syndrome)
- Exacerbation of Porphyria
- Teratogenicity and Other Harm to the Newborn
- Hyperglycemia
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The more important adverse clinical reactions caused by the IV use of CEREBYX or phenytoin are cardiovascular collapse and/or CNS depression. Hypotension can occur when either drug is administered rapidly by the IV route. The rate of administration is very important; for CEREBYX, it should not exceed 150 mg PE/min
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of phenytoin or fosphenytoin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: Anaphylaxis, angioedema [see
Hematologic: Pure red cell aplasia [see
Laboratory Test Abnormality: Phenytoin or CEREBYX may decrease serum concentrations of T4. It may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may also cause increased serum levels of gamma glutamyl transpeptidase (GGT).
Nervous System Disorders: Dyskinesia
5DRUG INTERACTIONS
Fosphenytoin is extensively bound to human plasma proteins. Drugs highly bound to albumin could increase the unbound fraction of fosphenytoin. Although, it is unknown whether this could result in clinically significant effects, caution is advised when administering CEREBYX with other drugs that significantly bind to serum albumin. The most significant drug interactions following administration of CEREBYX are expected to occur with drugs that interact with phenytoin. Phenytoin is extensively bound to serum plasma proteins and is prone to competitive displacement. Phenytoin is primarily metabolized by the hepatic cytochrome P450 enzyme CYP2C9 and to a lesser extent by CYP2C19 and is particularly susceptible to inhibitory drug interactions because it is subject to saturable metabolism. Inhibition of metabolism may produce significant increases in circulating phenytoin concentrations and enhance the risk of drug toxicity. Monitoring of phenytoin serum levels is recommended when a drug interaction is suspected.
Phenytoin or CEREBYX is a potent inducer of hepatic drug-metabolizing enzymes.
5.1Drugs that Affect Phenytoin or CEREBYX
Table 6 includes commonly occurring drug interactions that affect phenytoin (the active metabolite of CEREBYX) concentrations. However, this list is not intended to be inclusive or comprehensive. Individual prescribing information from relevant drugs should be consulted.
The addition or withdrawal of these agents in patients on phenytoin therapy may require an adjustment of the phenytoin dose to achieve optimal clinical outcome.
5.2Drugs Affected by Phenytoin or CEREBYX
Table 7 includes commonly occurring drug interactions affected by phenytoin (the active metabolite of CEREBYX). However, this list is not intended to be inclusive or comprehensive. Individual drug package inserts should be consulted. The addition or withdrawal of phenytoin during concomitant therapy with these agents may require adjustment of the dose of these agents to achieve optimal clinical outcome.
5.3Hyperammonemia with Concomitant Use of Valproate
Concomitant administration of phenytoin and valproate has been associated with an increased risk of valproate-associated hyperammonemia. Patients treated concomitantly with these two drugs should be monitored for signs and symptoms of hyperammonemia.
5.4Drug/Laboratory Test Interactions
Care should be taken when using immunoanalytical methods to measure serum phenytoin concentrations following CEREBYX administration.
6OVERDOSAGE
Nausea, vomiting, lethargy, tachycardia, bradycardia, asystole, cardiac arrest, hypotension, syncope, hypocalcemia, metabolic acidosis, and death have been reported in cases of overdosage with CEREBYX.
Because CEREBYX is a prodrug of phenytoin, the following information about phenytoin overdosage may be helpful. Initial symptoms of acute phenytoin toxicity are nystagmus, ataxia, and dysarthria. Other signs include tremor, hyperreflexia, lethargy, slurred speech, nausea, vomiting, coma, and hypotension. Death is caused by respiratory and circulatory depression. The lethal dose of phenytoin in adults is estimated to be 2 to 5 grams. The lethal dose in pediatrics is not known.
There are marked variations among individuals with respect to serum phenytoin concentrations where toxicity occurs. Lateral gaze nystagmus usually appears at 20 µg/mL, ataxia at 30 µg/mL, and dysarthria and lethargy appear when the serum concentration is over 40 µg/mL. However, phenytoin concentrations as high as 50 µg/mL have been reported without evidence of toxicity. As much as 25 times the therapeutic phenytoin dose has been taken, resulting in serum phenytoin concentrations over 100 µg/mL, with complete recovery. Irreversible cerebellar dysfunction and atrophy have been reported after overdosage.
Formate and phosphate are metabolites of CEREBYX and therefore may contribute to signs of toxicity following overdosage. Signs of formate toxicity are similar to those of methanol toxicity and are associated with severe anion-gap metabolic acidosis. Large amounts of phosphate, delivered rapidly, could potentially cause hypocalcemia with paresthesia, muscle spasms, and seizures. Ionized free calcium levels can be measured and, if low, used to guide treatment.
7DESCRIPTION
CEREBYX
The pharmacological class of the fosphenytoin sodium is hydantoin derivative, and the therapeutic class is anticonvulsant.
CEREBYX is marketed in 2 mL vials containing a total of 100 mg PE/2 mL (50 mg PE/mL) and 10 mL vials containing a total of 500 mg PE/10 mL (50 mg PE/mL), for intravenous or intramuscular administration. The concentration of each vial is 50 mg PE/mL. CEREBYX is supplied in vials as a sterile solution in Water for Injection, USP, and Tromethamine, USP (TRIS) (12.11 mg/mL), buffer adjusted to pH 8.6 to 9.0 with either Hydrochloric Acid, NF, or Sodium Hydroxide, NF. CEREBYX is a clear, colorless to pale yellow, sterile solution.
The chemical name of fosphenytoin is 5,5-diphenyl-3-[(phosphonooxy)methyl]-2,4-imidazolidinedione disodium salt. The molecular structure of fosphenytoin is:
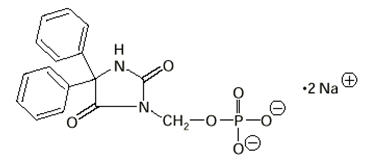
The molecular weight of fosphenytoin is 406.24.
8CLINICAL STUDIES
Infusion tolerance was evaluated in clinical studies. One double-blind study assessed infusion-site tolerance of equivalent loading doses (15 to 20 mg PE/kg) of CEREBYX infused at 150 mg PE/min or phenytoin infused at 50 mg/min. The study demonstrated better local tolerance (pain and burning at the infusion site), fewer disruptions of the infusion, and a shorter infusion period for CEREBYX-treated patients (Table 8).
CEREBYX-treated patients, however, experienced more systemic sensory disturbances
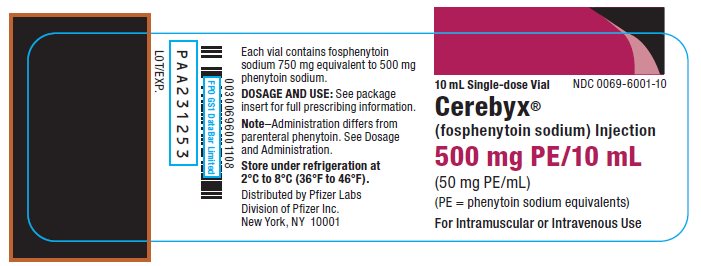
9PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
10 mL Single-dose Vial
NDC 0069-6001-10
NDC 0069-6001-10
Cerebyx
(fosphenytoin sodium) Injection
(fosphenytoin sodium) Injection
500 mg PE/10 mL
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)
For Intramuscular or Intravenous Use
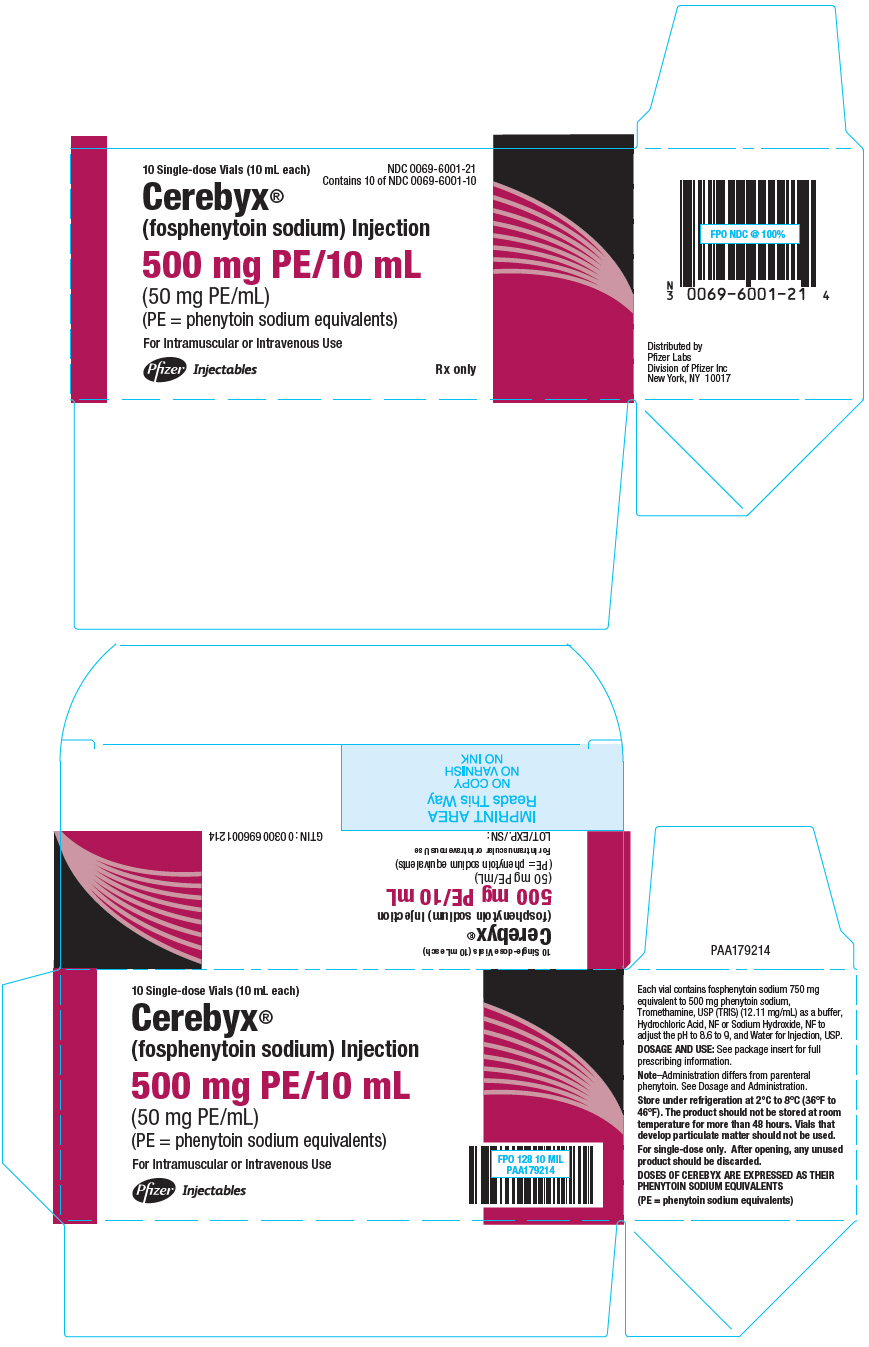
10PRINCIPAL DISPLAY PANEL - 10 mL Vial Package
10 Single-dose Vials (10 mL each)
NDC 0069-6001-21
Cerebyx
(fosphenytoin sodium) Injection
(fosphenytoin sodium) Injection
500 mg PE/10 mL
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)
For Intramuscular or Intravenous Use
Pfizer Hospital
Rx only
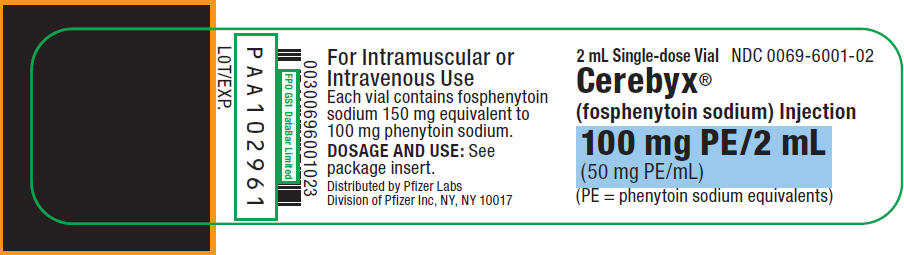
11PRINCIPAL DISPLAY PANEL - 2 mL Vial Label
2 mL Single-dose Vial
Cerebyx
100 mg PE/2 mL
(PE = phenytoin sodium equivalents)
12PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton
25 Single-dose Vials (2 mL each)
NDC 0069-6001-25
Cerebyx
(fosphenytoin sodium) Injection
(fosphenytoin sodium) Injection
100 mg PE/2 mL
(50 mg PE/mL)
(50 mg PE/mL)
(PE = phenytoin sodium equivalents)
For Intramuscular or Intravenous Use
Pfizer Hospital
Rx only

