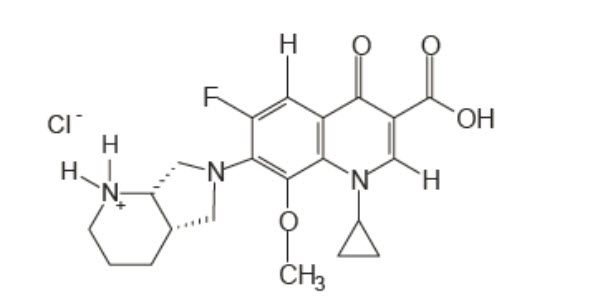
Moxifloxacin
What is Vigamox (Moxifloxacin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: While drug-susceptible tuberculosis (TB) disease in children currently requires four to six months of treatment, most children may be able to be cured with a shorter treatment of more powerful drugs. Shorter treatment may be easier for children to tolerate and finish as well as ease caregiver strain from managing treatment side effects and supporting children over many months. The primary objectiv...
Summary: This is a clinical trial looking at the impact of energy drinks on heart related parameters. The study will enroll 3 participants who will be exposed to 8 different interventions.
Summary: This is a phase 2B/C, open label platform study that will compare the efficacy, safety of experimental regimens with a standard control regimen in participants with newly diagnosed, drug sensitive pulmonary tuberculosis. In stage 1, participants will be randomly allocated to the control or one of the 2 rifampicin-containing experimental regimens in the ratio 1:1:1. In stage 2, the experimental arm...
Related Latest Advances
Brand Information
Micrococcus luteus*
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus warneri*
Streptococcus pneumoniae
Streptococcus viridansgroup
Acinetobacter lwoffii*
Haemophilus influenza
Haemophilus parainfluenzae*
Chlamydia trachomatis