Columvi
What is Columvi (Glofitamab)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of the study is to evaluate glofitamab + gemcitabine + oxaliplatin in participants in the United States, including under-represented racial and ethnic populations, that have relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL).
Summary: To learn if the combination of axicabtagene ciloleucel (axi-cel) and glofitamab as first-line therapy in high-risk LBCL participants or as second-line therapy in LBCL participants can help to control the disease.
Summary: The main goal of this trial is to study the frequency and severity of cytokine release syndrome (CRS) in participants with diffuse large B-cell lymphoma (DLBCL) who are using a combination of glofitamab + gemcitabine + oxaliplatin (Glofit-GemOx) followed by glofitamab-only treatment.
Related Latest Advances
Brand Information
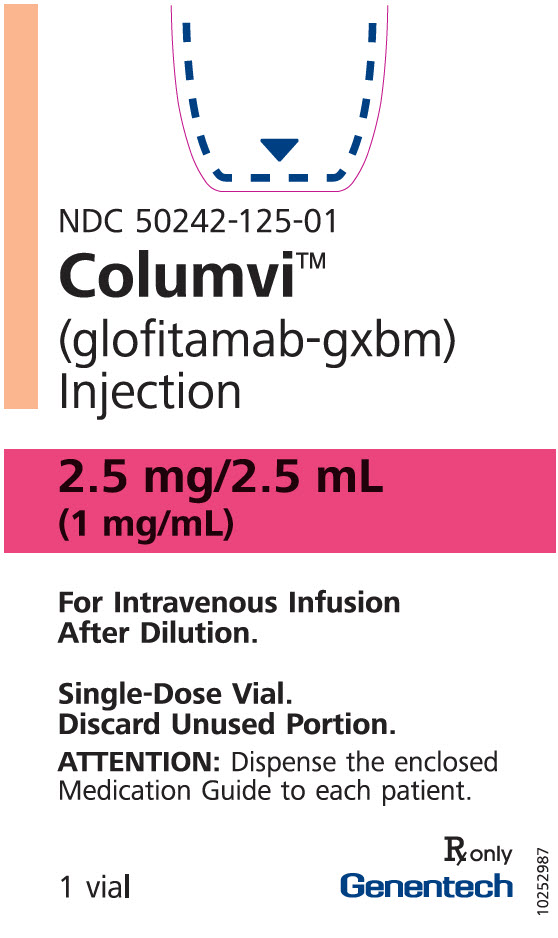
- 2.5 mg/2.5 mL (1 mg/mL) clear, colorless solution in a single-dose vial.
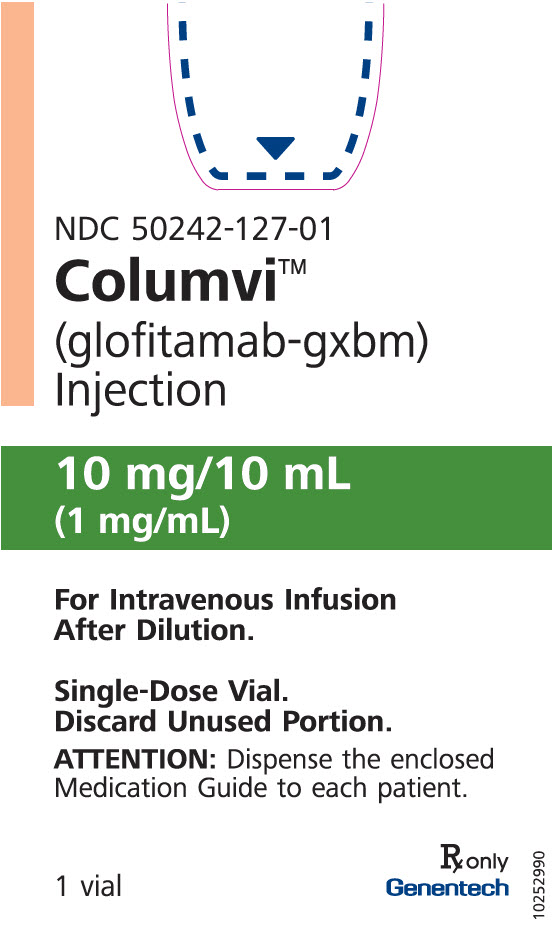
- 10 mg/10 mL (1 mg/mL) clear, colorless solution in a single-dose vial.
- Cytokine Release Syndrome
- Neurologic Toxicity
- Serious Infections
- Tumor Flare