Generic Name
Ivacaftor
Brand Names
Symdeko, Kalydeco, Trikafta, Orkambi
FDA approval date: January 31, 2012
Classification: Cystic Fibrosis Transmembrane Conductance Regulator Potentiator
Form: Tablet, Kit, Granule
What is Symdeko (Ivacaftor)?
TRIKAFTA is indicated for the treatment of cystic fibrosis in patients aged 2 years and older who have at least one F508del mutation in the cystic fibrosis transmembrane conductance regulator gene or a mutation in the CFTR gene that is responsive based on clinical and/or in vitro data [see Clinical Pharmacology (1.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 2a Randomized, Double-blind, Placebo-controlled Proof-of-concept Study to Evaluate the Safety, Tolerability, Pharmacodynamics, and Pharmacokinetics of SION-719 When Added to Physician-prescribed Trikafta® in People With Cystic Fibrosis Who Are Homozygous for the F508del Mutation
Summary: The purpose of this study is to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SION-719 when given to people with CF who are already taking Trikafta.
A Phase 1, Study of VX-828 in Healthy Subjects and in Subjects With Cystic Fibrosis
Summary: The purpose of the study is to evaluate safety, tolerability, and pharmacokinetics of VX-828 and VX-828 in triple combination (TC) with Tezacaftor (TEZ)/ VX-118 or TEZ/ deutivacaftor (D-IVA) in healthy participants and VX-828 in combination with D-IVA with or without TEZ in participants with cystic fibrosis (CF).
A Phase 1/2, Multicenter Study Evaluating the Safety, Tolerability, and Biodistribution of RCT2100 With Single-Ascending Doses in Healthy Participants and Multiple-Ascending Doses and Proof-of-Concept in Participants With Cystic Fibrosis
Summary: This is the first-in-human study with RCT2100 and is designed to provide safety and tolerability data for future clinical studies.
Related Latest Advances
Brand Information
SYMDEKO (Tezacaftor and Ivacaftor)
1INDICATIONS AND USAGE
SYMDEKO is indicated for the treatment of cystic fibrosis (CF) in patients aged 6 years and older who are homozygous for the
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a
2DOSAGE FORMS AND STRENGTHS
Tablets: Tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablets co-packaged with ivacaftor 75 mg tablets
- Tezacaftor 50 mg/ivacaftor 75 mg tablets are white, oblong-shaped, and debossed with "V50" on one side and plain on the other.
- Ivacaftor 75 mg tablets are light blue, oblong-shaped, and printed with "V 75" in black ink on one side and plain on the other.
Tablets: Tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets co-packaged with ivacaftor 150 mg tablets
- Tezacaftor 100 mg/ivacaftor 150 mg tablets are yellow, oblong-shaped, and debossed with "V100" on one side and plain on the other.
- Ivacaftor 150 mg tablets are light blue, oblong-shaped, and printed with "V 150" in black ink on one side and plain on the other.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Transaminase Elevations
- Hypersensitivity Reactions, Including Anaphylaxis
- Intracranial Hypertension
- Cataracts
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety profile of SYMDEKO is based on data from 1001 patients in three double-blind, placebo-controlled, clinical trials: two parallel-group trials of 12 and 24-week duration and one cross-over design trial of 8 weeks duration. Eligible patients were also able to participate in an open-label extension safety study (up to 96 weeks of SYMDEKO). In the three placebo-controlled trials (Trials 1, 2, and 3), a total of 496 patients with CF aged 12 years and older received at least one dose of SYMDEKO. The proportion of patients who discontinued study drug prematurely due to adverse reactions was 1.6% for SYMDEKO-treated patients and 2.0% for placebo-treated patients. Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in SYMDEKO-treated patients compared to placebo included distal intestinal obstruction syndrome, 3 (0.6%) SYMDEKO-treated patients vs. 0 placebo. There were no deaths in the placebo-controlled trials, and one death in the open-label extension study due to respiratory failure and influenza infection in a patient who had discontinued SYMDEKO seven weeks prior.
The safety profile of SYMDEKO was generally similar across all subgroups of patients, including analysis by age, sex, baseline percent predicted FEV
Table 5 shows adverse reactions occurring in ≥3% of SYMDEKO-treated patients that also occurred at a higher rate than in the placebo-treated patients in the 12- and 24-week placebo-controlled, parallel-group trials (Trials 1 and 3).
The safety data from the following trials are similar to that observed in Trials 1 and 3:
- an 8-week randomized, double-blind, placebo-controlled crossover study in 244 patients with CF aged 12 years and older who were heterozygous for the
- a 24-week open-label study in 70 patients with CF aged 6 to less than 12 years who were either homozygous for the
4.2Postmarketing Experience
Postmarketing Adverse Reactions with SYMDEKO
The following adverse reactions have been identified during post approval use of SYMDEKO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: anaphylaxis
Skin: rash
Postmarketing Adverse Reactions with Other Drugs Containing the Same or Similar Active Ingredients as SYMDEKO
The following adverse reactions have been identified during post approval use of drugs containing the same or similar active ingredients as SYMDEKO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous System Disorders: intracranial hypertension
5DRUG INTERACTIONS
Potential for other drugs to affect tezacaftor/ivacaftor
5.1Inducers of CYP3A
Tezacaftor and ivacaftor are substrates of CYP3A (ivacaftor is a sensitive substrate of CYP3A). Concomitant use of CYP3A inducers may result in reduced exposures and thus reduced SYMDEKO efficacy. Co-administration of ivacaftor with rifampin, a strong CYP3A inducer, significantly decreased ivacaftor exposure (area under the curve [AUC]) by 89%. Tezacaftor exposures can also be expected to decrease significantly during co-administration with strong CYP3A inducers. Therefore, co-administration of SYMDEKO with strong CYP3A inducers is not recommended
Examples of strong CYP3A inducers include:
- rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's wort (Hypericum perforatum)
5.2Inhibitors of CYP3A
Co-administration with itraconazole, a strong CYP3A inhibitor, increased tezacaftor exposure (AUC) by 4.0-fold and ivacaftor by 15.6-fold. When co-administered with strong CYP3A inhibitors, the dosing regimen of SYMDEKO should be adjusted
Examples of strong CYP3A inhibitors include:
- ketoconazole, itraconazole, posaconazole, and voriconazole
- telithromycin and clarithromycin
Co-administration of fluconazole increased ivacaftor exposure (AUC) by 3.0-fold. Simulation suggested co-administration with fluconazole, a moderate CYP3A inhibitor, may increase tezacaftor exposure (AUC) by approximately 2.0-fold. When co-administered with moderate CYP3A inhibitors, the dosing regimen of SYMDEKO should be adjusted
Examples of moderate CYP3A inhibitors include:
- fluconazole
- erythromycin
Co-administration of SYMDEKO with grapefruit juice, which contains one or more components that moderately inhibit CYP3A, may increase exposure of tezacaftor and ivacaftor; therefore, food or drink containing grapefruit should be avoided during treatment with SYMDEKO
5.3Ciprofloxacin
Co-administration of SYMDEKO with ciprofloxacin had no significant effect on the exposure of tezacaftor or ivacaftor. Therefore, no dosage adjustment is necessary during concomitant administration of SYMDEKO with ciprofloxacin
Potential for tezacaftor/ivacaftor to affect other drugs
5.4CYP3A Substrates
Co-administration of SYMDEKO with midazolam (oral), a sensitive CYP3A substrate, did not affect midazolam exposure. No dosage adjustment of CYP3A substrates is required when co-administered with SYMDEKO
5.5CYP2C9 Substrates
Ivacaftor may inhibit CYP2C9; therefore, monitoring of the international normalized ratio (INR) during co-administration of SYMDEKO with warfarin is recommended. Other medicinal products for which exposure may be increased by SYMDEKO include glimepiride and glipizide; these medicinal products should be used with caution
5.6Digoxin and Other P-gp Substrates
Co-administration of SYMDEKO with digoxin, a sensitive P-gp substrate, increased digoxin exposure by 1.3-fold consistent with weak inhibition of P-gp by ivacaftor. Administration of SYMDEKO may increase systemic exposure of medicinal products that are sensitive substrates of P-gp, which may increase or prolong their therapeutic effect and adverse reactions. When used concomitantly with digoxin or other substrates of P-gp with a narrow therapeutic index such as cyclosporine, everolimus, sirolimus, and tacrolimus, caution and appropriate monitoring should be used
5.7Hormonal Contraceptives
SYMDEKO has been studied with an ethinyl estradiol/norethindrone oral contraceptive and was found to have no significant effect on the exposures of the hormonal contraceptive. SYMDEKO is not expected to modify the efficacy of hormonal contraceptives
6OVERDOSAGE
No specific antidote is available for overdose with SYMDEKO. Treatment of overdosage consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
7DESCRIPTION
SYMDEKO is co-packaged as a tezacaftor/ivacaftor fixed-dose combination tablet and an ivacaftor tablet. Both tablets are for oral administration.
8HOW SUPPLIED/STORAGE AND HANDLING
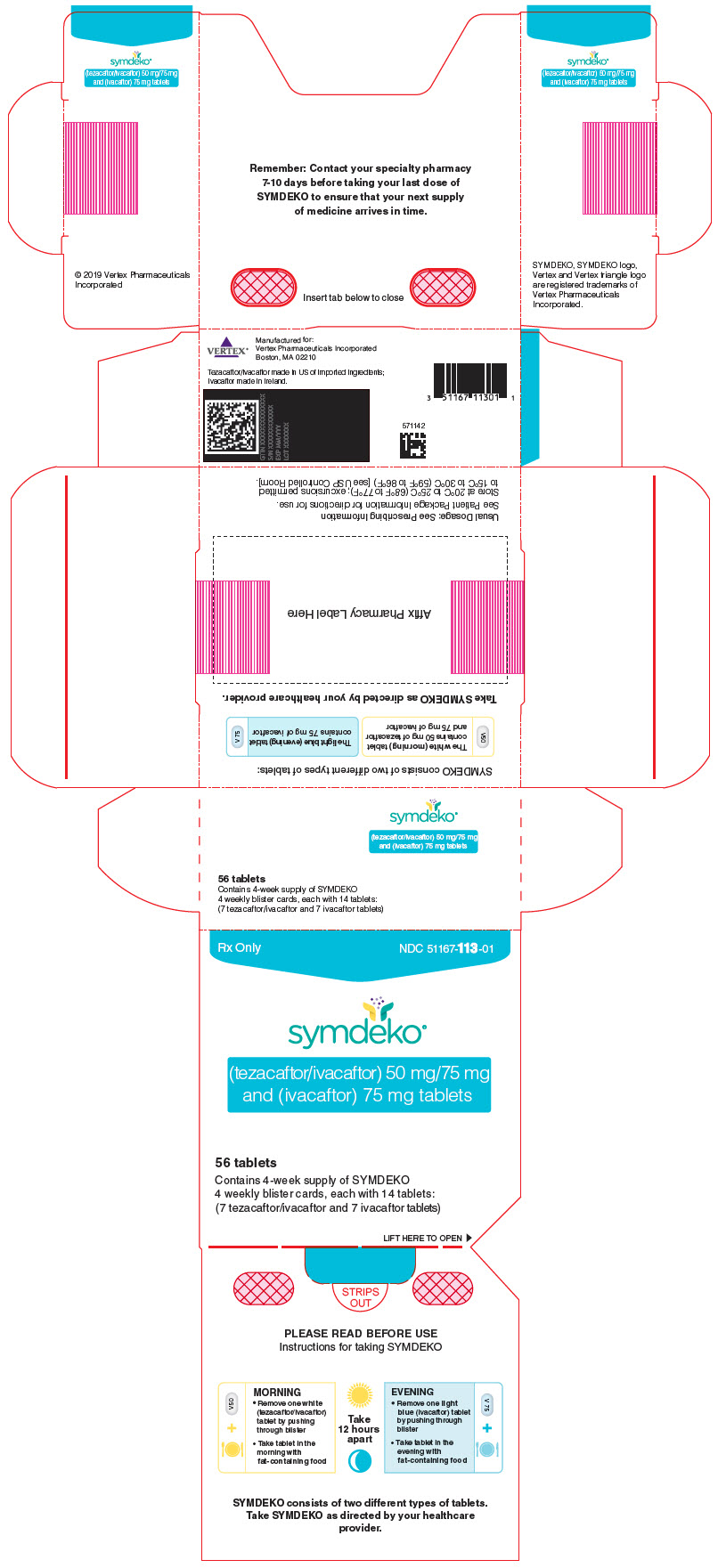
SYMDEKO (tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablets co-packaged with ivacaftor 75 mg tablet):
- Tezacaftor 50 mg/ivacaftor 75 mg fixed-dose combination tablets are supplied as white, oblong-shaped tablets containing 50 mg of tezacaftor and 75 mg of ivacaftor. Each tablet is debossed with "V50" on one side and plain on the other.
- Ivacaftor 75 mg tablets are supplied as light blue, film-coated, oblong-shaped tablets containing 75 mg of ivacaftor. Each tablet is printed with the characters "V 75" on one side and plain on the other.
- 56-count tablet carton containing a 4-week supply (4 weekly wallets, each with 14 tablets)
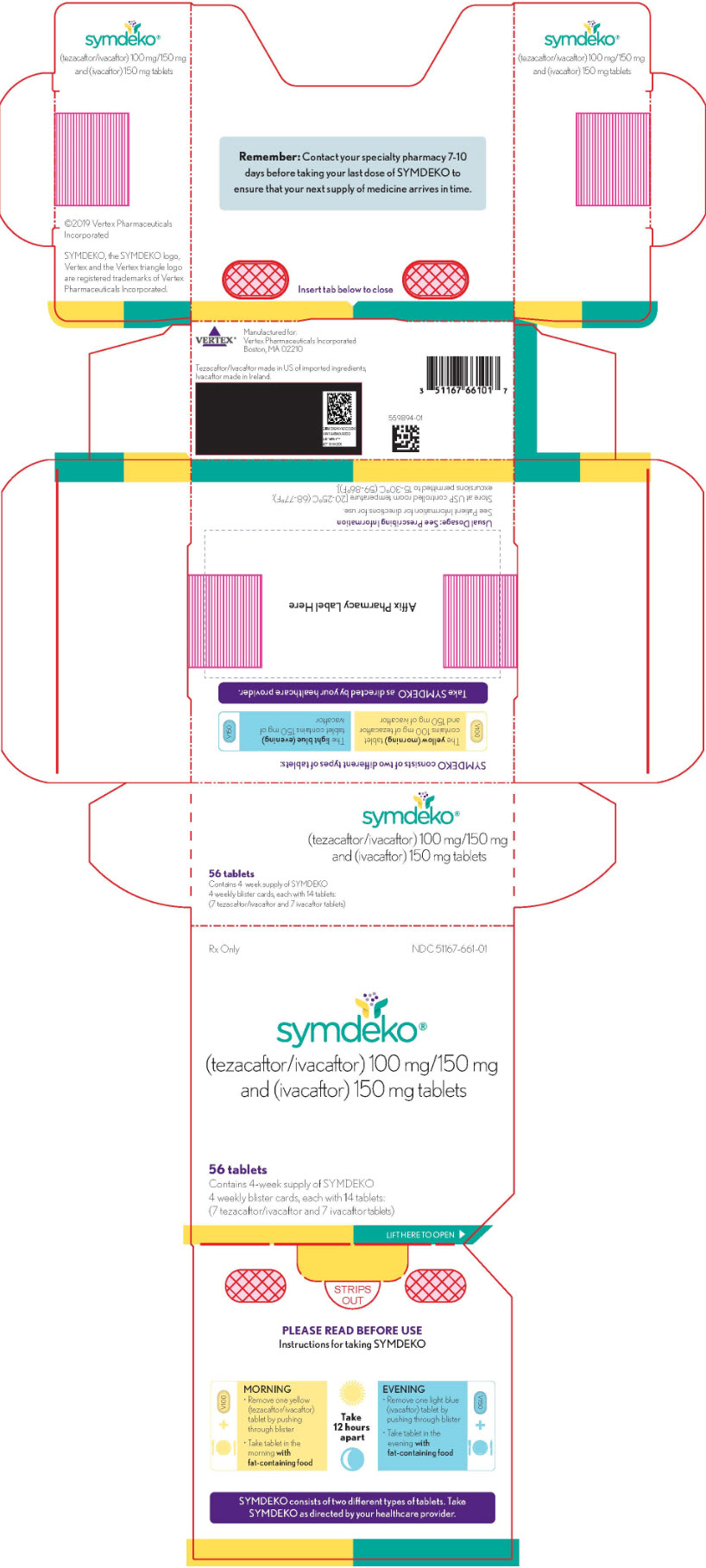
SYMDEKO (tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets co-packaged with ivacaftor 150 mg tablet):
- Tezacaftor 100 mg/ivacaftor 150 mg fixed-dose combination tablets are supplied as yellow, oblong-shaped tablets containing 100 mg of tezacaftor and 150 mg of ivacaftor. Each tablet is debossed with "V100" on one side and plain on the other.
- Ivacaftor 150 mg tablets are supplied as light blue, film-coated, oblong-shaped tablets containing 150 mg of ivacaftor. Each tablet is printed with the characters "V 150" on one side and plain on the other.
- 56-count tablet carton containing a 4-week supply (4 weekly wallets, each with 14 tablets)
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
10PRINCIPAL DISPLAY PANEL - Kit Carton
Rx Only
symdeko
56 tablets
LIFT HERE TO OPEN ▶
11PRINCIPAL DISPLAY PANEL - Kit Carton - 113
Rx Only
symdeko
(tezacaftor/ivacaftor) 50 mg/75 mg
56 tablets
Contains 4-week supply of SYMDEKO
LIFT HERE TO OPEN ▶