Brand Name
Multaq
Generic Name
Dronedarone
View Brand Information FDA approval date: July 01, 2009
Classification: Antiarrhythmic
Form: Tablet
What is Multaq (Dronedarone)?
MULTAQ ® is indicated to reduce the risk of hospitalization for atrial fibrillation in patients in sinus rhythm with a history of paroxysmal or persistent atrial fibrillation . MULTAQ is an antiarrhythmic drug indicated to reduce the risk of hospitalization for atrial fibrillation in patients in sinus rhythm with a history of paroxysmal or persistent AF .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Multaq (Dronedarone)
WARNING: INCREASED RISK OF DEATH, STROKE AND HEART FAILURE IN PATIENTS WITH DECOMPENSATED HEART FAILURE OR PERMANENT ATRIAL FIBRILLATION
In patients with symptomatic heart failure and recent decompensation requiring hospitalization or NYHA Class IV heart failure, MULTAQ doubles the risk of death
In patients with permanent atrial fibrillation, MULTAQ doubles the risk of death, stroke and hospitalization for heart failure
1INDICATIONS AND USAGE
MULTAQ
2DOSAGE AND ADMINISTRATION
The recommended dosage of MULTAQ is 400 mg twice daily in adults. MULTAQ should be taken as one tablet with the morning meal and one tablet with the evening meal.
Treatment with Class I or III antiarrhythmics (e.g., amiodarone, flecainide, propafenone, quinidine, disopyramide, dofetilide, sotalol) or drugs that are strong inhibitors of CYP3A (e.g., ketoconazole) must be stopped before starting MULTAQ
Verify that females of reproductive potential are not pregnant prior to initiating MULTAQ
3DOSAGE FORMS AND STRENGTHS
MULTAQ 400 mg tablets are provided as white film-coated tablets for oral administration, oblong-shaped, engraved with a double wave marking on one side and "4142" code on the other side.
4CONTRAINDICATIONS
MULTAQ is contraindicated in patients with:
- Permanent atrial fibrillation (patients in whom normal sinus rhythm will not or cannot be restored)
- Symptomatic heart failure with recent decompensation requiring hospitalization or NYHA Class IV symptoms
- Second or third-degree atrioventricular (AV) block, or sick sinus syndrome (except when used in conjunction with a functioning pacemaker)
- Bradycardia <50 bpm
- Concomitant use of strong CYP3A inhibitors, such as ketoconazole, itraconazole, voriconazole, cyclosporine, telithromycin, clarithromycin, nefazodone, and ritonavir
- Concomitant use of erythromycin
- Concomitant use of drugs or herbal products that prolong the QT interval and might increase the risk of torsade de pointes, such as phenothiazine antipsychotics, tricyclic antidepressants, certain oral macrolide antibiotics, and Class I and III antiarrhythmics
- Liver or lung toxicity related to the previous use of amiodarone
- QTc interval >500 ms or PR interval >280 ms
- Severe hepatic impairment
- Hypersensitivity to the active substance or to any of the excipients
5ADVERSE REACTIONS
The following safety concerns are described elsewhere in the label:
- New or worsening heart failure
- Liver Injury
- Pulmonary toxicity
- Hypokalemia and hypomagnesemia with potassium-depleting diuretics
- QT prolongation
5.1Clinical Trials Experience
The safety evaluation of dronedarone 400 mg twice daily in patients with AF or AFL is based on 5 placebo-controlled studies, ATHENA, EURIDIS, ADONIS, ERATO and DAFNE. In these studies, a total of 6285 patients were randomized and treated, 3282 patients with MULTAQ 400 mg twice daily, and 2875 with placebo. The mean exposure across studies was 12 months. In ATHENA, the maximum follow-up was 30 months.
In clinical trials, premature discontinuation because of adverse reactions occurred in 11.8% of the dronedarone-treated patients and in 7.7% of the placebo-treated group. The most common reasons for discontinuation of therapy with MULTAQ were gastrointestinal disorders (3.2% vs 1.8% in the placebo group) and QT prolongation (1.5% vs 0.5% in the placebo group).
The most frequent adverse reactions observed with MULTAQ 400 mg twice daily in the 5 studies were diarrhea, nausea, abdominal pain, vomiting, and asthenia.
Table 1 displays adverse reactions more common with dronedarone 400 mg twice daily than with placebo in AF or AFL patients, presented by system organ class and by decreasing order of frequency. Adverse laboratory and ECG effects are presented separately in Table 2.
Photosensitivity reaction and dysgeusia have also been reported at an incidence less than 1% in patients treated with MULTAQ.
The following laboratory data/ECG parameters were reported with MULTAQ 400 mg twice daily.
Assessment of demographic factors such as gender or age on the incidence of treatment-emergent adverse events did not suggest an excess of adverse events in any particular subgroup.
In randomized clinical trials of patients with paroxysmal or persistent atrial fibrillation, one case of torsade de pointes was reported in patients treated with MULTAQ (2301 patients) versus no cases of torsade de pointes in patients treated with placebo (2327) in the ATHENA study. No cases of torsade de pointes were reported in patients treated with MULTAQ (828 patients) or placebo (409 patients) in the EURIDIS and ADONIS studies
5.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of MULTAQ. Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac: New or worsening heart failure [see
Atrial flutter with 1:1 atrioventricular conduction has been reported very rarely.
Hepatic: Liver injury [see
Respiratory: Interstitial lung disease including pneumonitis and pulmonary fibrosis [see
Immune: Anaphylactic reactions including angioedema
Vascular: Vasculitis, including leukocytoclastic vasculitis
6OVERDOSAGE
In the event of overdosage, monitor the patient's cardiac rhythm and blood pressure. Treatment should be supportive and based on symptoms.
It is not known whether dronedarone or its metabolites can be removed by dialysis (hemodialysis, peritoneal dialysis or hemofiltration). There is no specific antidote available.
7DESCRIPTION
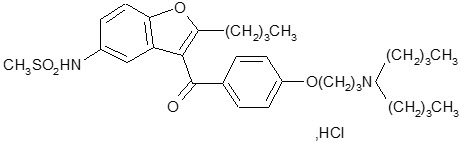
Dronedarone HCl is a benzofuran derivative with the following chemical name:
N-{2-butyl-3-[4-(3-dibutylaminopropoxy)benzoyl]benzofuran-5-yl} methanesulfonamide, hydrochloride.
Dronedarone HCl is a white fine powder that is practically insoluble in water and freely soluble in methylene chloride and methanol.
Its empirical formula is C
MULTAQ is provided as tablets for oral administration.
Each tablet of MULTAQ contains 400 mg of dronedarone (expressed as base).
The inactive ingredients are:
- Core of the tablets: Colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, poloxamer 407, starch.
- Coating/polishing of the tablets: Carnauba wax, hypromellose, polyethylene glycol 6000, titanium dioxide.
8HOW SUPPLIED/STORAGE AND HANDLING
MULTAQ 400-mg tablets are provided as white film-coated tablets for oral administration, oblong-shaped, engraved with a double wave marking on one side and "4142" code on the other side in:
- Bottles of approximately 1260 film-coated tablets, NDC 55154-8104-2
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
10Medication Guide
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised May 2025
11Package/Label Display Panel
Contains Approximately
1260 Tablets
MULTAQ®
(dronedarone) Tablets
400 mg
Each tablet contains:
dronedarone hydrochloride equivalent to
400mg dronedarone.
Dispense with Medication Guide.
Rx Only
WARNING: Keep out of reach of
Children.
