Brand Name
Aldurazyme
Generic Name
Laronidase
View Brand Information FDA approval date: April 30, 2003
Classification: Hydrolytic Lysosomal Glycosaminoglycan-specific Enzyme
Form: Injection
What is Aldurazyme (Laronidase)?
ALDURAZYME ® is indicated for adult and pediatric patients with Hurler and Hurler-Scheie forms of Mucopolysaccharidosis I and for patients with the Scheie form who have moderate to severe symptoms. Limitations of Use: The risks and benefits of treating mildly affected patients with the Scheie form have not been established. ALDURAZYME has not been evaluated for effects on the central nervous system manifestations of the disorder. ALDURAZYME is a hydrolytic lysosomal glycosaminoglycan -specific enzyme indicated for adult and pediatric patients with Hurler and Hurler-Scheie forms of Mucopolysaccharidosis I and for patients with the Scheie form who have moderate to severe symptoms. Limitations of Use: The risks and benefits of treating mildly affected patients with the Scheie form have not been established. ALDURAZYME has not been evaluated for effects on the central nervous system manifestations of the disorder.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Mucopolysaccharidosis I (MPS I) Registry
Summary: The Mucopolysaccharidosis I (MPS I) Registry is an ongoing, observational database that tracks the outcomes of patients with MPS I. The data collected by the MPS I Registry will provide information to better characterize the natural history and progression of MPS I as well as the clinical responses of patients receiving enzyme replacement therapy, such as Aldurazyme (Recombinant Human Alpha-L-Idur...
PEARL (PrEnAtal Enzyme Replacement Therapy for Lysosomal Storage Disorders)
Summary: For detailed information, please view our study website: https://pearltrial.ucsf.edu/ The investigators aims to determine the the maternal and fetal safety and feasibility of in utero fetal enzyme replacement therapy in fetuses with Lysosomal Storage Diseases.
Related Latest Advances
Brand Information
ALDURAZYME (laronidase)
1INDICATIONS AND USAGE
ALDURAZYME
- adult and pediatric patients with Hurler and Hurler-Scheie forms of Mucopolysaccharidosis I (MPS I) and
- patients with the Scheie form of MPS I who have moderate to severe symptoms.
2DOSAGE FORMS AND STRENGTHS
Injection: 2.9 mg/5 mL (0.58 mg/mL) of laronidase as a colorless to pale yellow, clear to slightly opalescent solution in a single-dose vial.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
Serious and or clinically significant adverse reactions described elsewhere in labeling include:
- Hypersensitivity Reactions Including Anaphylaxis
- Acute Respiratory Complications Associated with Administration
- Acute Cardiorespiratory Failure
- Infusion-Associated Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Serious adverse reactions reported with ALDURAZYME treatment during clinical trials were anaphylactic and hypersensitivity reactions. The most common adverse reactions were infusion reactions. The frequency of infusion reactions decreased over time with continued use of ALDURAZYME, and the majority of reactions were classified as being mild to moderate in severity.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of ALDURAZYME. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In postmarketing experience with ALDURAZYME, severe and serious infusion reactions have been reported, some of which were life-threatening, including anaphylactic shock
Adverse reactions resulting in death reported in the postmarketing setting with ALDURAZYME treatment included cardiorespiratory arrest, respiratory failure, cardiac failure, and pneumonia. These events have been reported in MPS I patients with underlying disease
Additional adverse reactions included fatigue, peripheral edema, erythema and cyanosis.
There have been a small number of reports of extravasation in patients treated with ALDURAZYME. There have been no reports of tissue necrosis associated with extravasation.
5DESCRIPTION
ALDURAZYME (laronidase) is a polymorphic variant of the human enzyme α-L-iduronidase that is produced by recombinant DNA technology in a Chinese hamster ovary cell line. α-L-iduronidase (glycosaminoglycan α-L-iduronohydrolase, EC 3.2.1.76) is a lysosomal hydrolase that catalyzes the hydrolysis of terminal α-L-iduronic acid residues of dermatan sulfate and heparan sulfate.
Laronidase is a glycoprotein with a molecular weight of approximately 83 kD. The predicted amino acid sequence of the recombinant form, as well as the nucleotide sequence that encodes it, are identical to a polymorphic form of human α-L-iduronidase. The recombinant protein is comprised of 628 amino acids after cleavage of the N-terminus and contains 6 N-linked oligosaccharide modification sites. Two oligosaccharide chains terminate in mannose-6-phosphate sugars. ALDURAZYME has a specific activity of approximately 172 U/mg.
ALDURAZYME, for intravenous infusion, is supplied as a sterile, nonpyrogenic, colorless to pale yellow, clear to slightly opalescent solution that must be diluted prior to administration in 0.9% Sodium Chloride Injection, USP. The solution in each vial contains a nominal laronidase concentration of 0.58 mg/mL and a pH of approximately 5.5. The extractable volume of 5 mL from each vial provides 2.9 mg laronidase, 43.9 mg sodium chloride, 63.5 mg sodium phosphate monobasic monohydrate, 10.7 mg sodium phosphate dibasic heptahydrate, and 0.05 mg polysorbate 80. ALDURAZYME does not contain preservatives; vials are for single dose only.
6HOW SUPPLIED/STORAGE AND HANDLING
ALDURAZYME (laronidase) injection is supplied as a colorless to pale yellow, clear to slightly opalescent solution in single-dose, clear Type I glass vial. Each vial contains 2.9 mg/5 mL (0.58 mg/mL) of laronidase. The closure consists of a siliconized butyl stopper and an aluminum seal with a plastic flip-off cap. ALDURAZYME is available as: One single-dose vial in a carton (NDC 58468-0070-1)
7PRINCIPAL DISPLAY PANEL - 2.9 mg/5 mL Vial Label
NDC 58468-0070-1
ALDURAZYME
2.9 mg/5 mL (0.58 mg/mL)
Injection
Rx Only

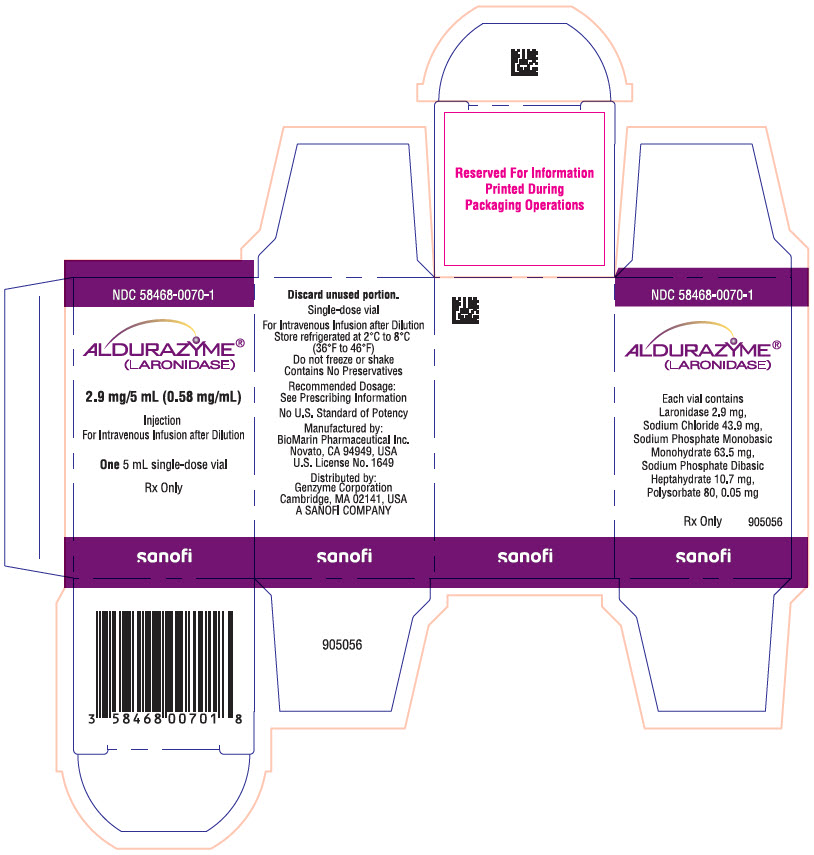
8PRINCIPAL DISPLAY PANEL - 2.9 mg/5 mL Vial Carton
NDC 58468-0070-1
ALDURAZYME
2.9 mg/5 mL (0.58 mg/mL)
Injection
One 5 mL single-dose vial
Rx Only
sanofi