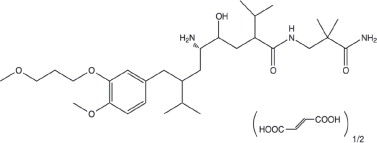
Tekturna
What is Tekturna (Aliskiren)?
High blood pressure, or hypertension, is often called the “silent killer” because it can quietly damage the heart, blood vessels, and kidneys for years before any symptoms appear. Managing blood pressure effectively is essential to prevent complications such as heart attack, stroke, and kidney disease. Tekturna (aliskiren) offers a modern approach to blood pressure control by targeting the body’s hormone system in a unique way.
Tekturna is a prescription medication used to treat high blood pressure in adults. It belongs to a class of drugs known as direct renin inhibitors (DRIs), the first of its kind approved by the U.S. Food and Drug Administration (FDA). Unlike other blood pressure medications that act later in the body’s renin-angiotensin system, Tekturna works at the very beginning of this pathway. This allows it to help control blood pressure by directly reducing the production of substances that cause blood vessels to tighten.
Since its approval, Tekturna has been recognized as an effective option for patients who may not respond well to other antihypertensive medications or who need additional support alongside other treatments.
What does Tekturna do?
Tekturna is prescribed to lower high blood pressure (hypertension) in adults. By helping to relax and widen blood vessels, it allows blood to flow more easily, which in turn reduces strain on the heart.
For many people, high blood pressure develops without obvious symptoms but increases the risk of serious health problems over time. When blood pressure is well controlled, the risks of heart attack, stroke, kidney damage, and heart failure are significantly reduced.
In clinical studies, Tekturna has been shown to effectively lower both systolic (top number) and diastolic (bottom number) blood pressure after several weeks of consistent use (FDA, 2024). It can be prescribed alone or in combination with other blood pressure medications such as diuretics or calcium channel blockers for better control.
Patients who take Tekturna as directed often notice more stable blood pressure readings and improved energy, helping them better manage their cardiovascular health over the long term.
How does Tekturna work?
Tekturna (aliskiren) works by blocking the activity of renin, an enzyme produced by the kidneys that plays a key role in blood pressure regulation.
Under normal circumstances, renin triggers a chain reaction known as the renin-angiotensin-aldosterone system (RAAS). This process leads to the production of angiotensin II, a hormone that tightens blood vessels and increases blood pressure. By inhibiting renin directly, Tekturna stops this chain reaction at its starting point.
As a result, blood vessels remain more relaxed and open, allowing blood to flow with less resistance. This lowers blood pressure and lowers the heart’s workload.
Clinically, this mechanism is important because it provides an additional therapeutic option for patients whose hypertension isn’t fully controlled by traditional medications like ACE inhibitors or angiotensin receptor blockers (ARBs). Unlike those drugs, Tekturna targets the very beginning of the hormonal process that raises blood pressure, offering a unique and effective way to keep it under control.
Tekturna side effects
Most people tolerate Tekturna well, but like any medication, it can cause side effects. Understanding what to expect helps patients use it safely and confidently.
Common side effects may include:
- Diarrhea
- Cough
- Dizziness or lightheadedness
- Headache
- Tiredness
These effects are usually mild and tend to improve as the body adjusts to the medication. Taking Tekturna with food may help reduce stomach-related symptoms.
Serious but less common side effects may include:
- Swelling of the face, lips, tongue, or throat (angioedema)
- Severe dizziness or fainting (possible low blood pressure)
- High potassium levels (hyperkalemia), which can cause muscle weakness or irregular heartbeat
- Kidney problems, especially in patients with preexisting kidney disease
Tekturna is contraindicated in pregnant women (fetal harm/death in 2nd/3rd trimester), diabetics using ACE inhibitors or ARBs (risk of kidney injury, hypotension, hyperkalemia), and individuals with severe kidney problems or aliskiren allergy.
Seek immediate medical help if you experience facial swelling, difficulty breathing, or chest pain after taking Tekturna. Regular blood pressure checks and lab tests are essential to ensure Tekturna remains safe and effective over time.
Tekturna dosage
Tekturna is a once-daily oral tablet. It takes 2-4 weeks for full effect, and consistent daily timing (with or without food) is important. Do not stop abruptly. Doctors may combine it with other blood pressure drugs, but avoid ACE inhibitors or ARBs in diabetics or those with kidney problems due to safety risks.
Routine monitoring for Tekturna includes blood pressure, kidney function, and potassium level checks. It’s generally safe for older adults and those with mild kidney issues with careful medical oversight.
Does Tekturna have a generic version?
Yes. A generic version of Tekturna (aliskiren) is now available in the United States and several other countries. Generic aliskiren tablets contain the same active ingredient, dosage strength, and quality as brand-name Tekturna.
FDA-approved generic medications are safe, pure, and effective, offering a reliable and affordable option. Tekturna is the original brand, while Tekturna HCT combines aliskiren with hydrochlorothiazide (a diuretic), beneficial for patients needing multiple medications to control blood pressure.
Conclusion
Tekturna (aliskiren) is a modern and effective option for controlling high blood pressure. By targeting renin, the enzyme that starts the chain reaction leading to vessel tightening, it offers a unique way to keep blood pressure within a healthy range.
While side effects can occur, monitoring and communication with your doctor ensure safe and effective treatment. With consistent use, a healthy lifestyle, and regular check-ups, Tekturna helps you live a longer, healthier, more balanced life.
References
- U.S. Food and Drug Administration (FDA). (2024). Tekturna (aliskiren) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Aliskiren (oral route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Aliskiren: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Antihypertensive medications and renin inhibition. Retrieved from https://www.nih.gov
Approved To Treat
Related Clinical Trials
Summary: The aim of this cross-over trial is to assess aliskiren, a direct renin inhibitor, as a novel treatment to block complement activation in the kidneys and thereby attenuate renal disease and stabilize or improve kidney function and compare it to the currently used treatment with the angiotensin converting enzyme inhibitor, enalapril, in patients with the complement-mediated renal disease C3 glomeru...
Related Latest Advances
Brand Information
- Fetal Toxicity
- Anaphylactic Reactions and Head and Neck Angioedema
- Hypotension