Brand Name
Luxturna
Generic Name
Voretigene Neparvovec-Rzyl
View Brand Information FDA approval date: December 19, 2017
Form: Kit
What is Luxturna (Voretigene Neparvovec-Rzyl)?
LUXTURNA is an adeno-associated virus vector-based gene therapy indicated for the treatment of patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy. Patients must have viable retinal cells as determined by the treating physician. LUXTURNA is an adeno-associated virus vector-based gene therapy indicated for the treatment of patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy. Patients must have viable retinal cells as determined by the treating physician.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
LUXTURNA (voretigene neparvovec-rzyl)
1INDICATIONS AND USAGE
LUXTURNA (voretigene neparvovec-rzyl) is an adeno-associated virus vector-based gene therapy indicated for the treatment of patients with confirmed biallelic
Patients must have viable retinal cells as determined by the treating physician(s).
2DOSAGE FORMS AND STRENGTHS
LUXTURNA is a suspension for subretinal injection, supplied in a 0.5-mL extractable volume in a 2-mL single-dose vial; the supplied concentration (5 x 10
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 5%) were conjunctival hyperemia, cataract, increased intraocular pressure, retinal tear, dellen (thinning of the corneal stroma), macular hole, subretinal deposits, eye inflammation, eye irritation, eye pain, and maculopathy (wrinkling on the surface of the macula).
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of other products and may not reflect the rates observed in practice.
The safety data described in this section reflect exposure to LUXTURNA in two clinical trials consisting of 41 subjects (81 eyes) with confirmed biallelic RPE65 mutation-associated retinal dystrophy. Forty of the 41 subjects received sequential subretinal injections of LUXTURNA to each eye. One subject received LUXTURNA in only one eye. Seventy-two of the 81 eyes were exposed to the recommended dose of LUXTURNA at 1.5 x 1011 vg; 9 eyes were exposed to lower doses of LUXTURNA. Study 1 (n=12) was an open-label, dose-exploration safety study. Study 2 (n=29) was an open-label, randomized, controlled study for both efficacy and safety [see Clinical Studies (]. The average age of the 41 subjects was 17 years, ranging from 4 to 44 years. Of the 41 subjects, 25 (61%) were pediatric subjects under 18 years of age, and 23 (56%) were females.
Twenty-seven (27/41, 66%) subjects had ocular adverse reactions that involved 46 injected eyes (46/81, 57%). Adverse reactions among all subjects in Studies 1 and 2 are described in Table 1. Adverse reactions may have been related to voretigene neparvovec-rzyl, the subretinal injection procedure, the concomitant use of corticosteroids, or a combination of these procedures and products.
*Transient appearance of asymptomatic subretinal precipitates inferior to the retinal injection site 1-6 days after injection
Immunogenicity
At all doses of LUXTURNA evaluated in Studies 1 and 2, immune reactions and extra-ocular exposure were mild. In Study 1 (n=12), the interval between the subretinal injections into the two eyes ranged from 1.7 to 4.6 years. In Study 2, the interval between the subretinal injections into the two eyes ranged from 7 to 14 days. No subject had a clinically significant cytotoxic T-cell response to either AAV2 or RPE65.
Subjects received systemic corticosteroids before and after subretinal injection of LUXTURNA to each eye. The corticosteroids may have decreased the potential immune reaction to either vector capsid (adeno-associated virus serotype 2 [AAV2] vector) or transgene product (retinoid isomerohydrolase RPE65 [RPE65]).
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of LUXTURNA. Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye Disorders: chorioretinal atrophy (also reported as retinal degeneration, retinal depigmentation, and injection site atrophy).
5DESCRIPTION
LUXTURNA (voretigene neparvovec-rzyl) is a suspension of an adeno-associated virus vector-based gene therapy for subretinal injection. LUXTURNA is a live, non-replicating adeno-associated virus serotype 2 which has been genetically modified to express the human
Each single-dose vial of LUXTURNA contains 5 x 1012 vector genomes (vg) per mL, and the excipients 180 mM sodium chloride, 10 mM sodium phosphate, and 0.001% Poloxamer 188 (pH 7.3), in a 0.5-mL extractable volume. LUXTURNA requires a 1:10 dilution prior to administration. After dilution, each dose of LUXTURNA consists of 1.5 x 1011 vg in a deliverable volume of 0.3 mL.
The Diluent, supplied in 1.7 mL extractable volume per vial in two 2-mL vials, is composed of sterile water containing 180 mM sodium chloride, 10 mM sodium phosphate, and 0.001% Poloxamer 188 (pH 7.3).
LUXTURNA may also contain residual components of HEK293 cells including DNA and protein and trace quantities of fetal bovine serum.
The product contains no preservative.
6CLINICAL STUDIES
The efficacy of LUXTURNA in pediatric and adult patients with biallelic RPE65 mutation-associated retinal dystrophy was evaluated in an open-label, two-center, randomized trial (Study 2). Of the 31 enrolled subjects, 21 subjects were randomized to receive subretinal injection of LUXTURNA. One subject discontinued from the study prior to treatment. Ten subjects were randomized to the control (non-intervention) group. One subject in the control group withdrew consent and was discontinued from the study. The nine subjects who were randomized to the control group were crossed over to receive subretinal injection of LUXTURNA after one year of observation. The average age of the 31 randomized subjects was 15 years (range 4 to 44 years), including 64% pediatric subjects (n=20, age from 4 to 17 years) and 36% adults (n=11). The 31 randomized subjects included 13 males and 18 females. Sixty-eight percent (68%) of the subjects were White, 16% were Asian, 10% were American Indian or Alaska Native, and 6% were Black or African-American. Bilateral subretinal injections of LUXTURNA were administered sequentially in two separate surgical procedures with an interval of 6 to 18 days.
The efficacy of LUXTURNA was established on the basis of multi-luminance mobility testing (MLMT) score change from Baseline to Year 1. The MLMT was designed to measure changes in functional vision, as assessed by the ability of a subject to navigate a course accurately and at a reasonable pace at different levels of environmental illumination. The MLMT was assessed using both eyes and each eye separately at one or more of seven levels of illumination, ranging from 400 lux (corresponding to a brightly lit office) to 1 lux (corresponding to a moonless summer night). Each light level was assigned a score code ranging from 0 to 6. A higher score indicated that a subject was able to pass the MLMT at a lower light level. A score of -1 was assigned to subjects who could not pass MLMT at a light level of 400 lux. The MLMT of each subject was videotaped and assessed by independent graders. The MLMT score was determined by the lowest light level at which the subject was able to pass the MLMT. The MLMT score change was defined as the difference between the score at Baseline and the score at Year 1. A positive MLMT score change from Baseline to Year 1 visit indicated that the subject was able to complete the MLMT at a lower light level.
Additional clinical outcomes were also evaluated, including full-field light sensitivity threshold (FST) testing, visual acuity, and visual fields.
Table 2 summarizes the median MLMT score change from Baseline to Year 1 in the LUXTURNA treatment group as compared to the control group. A median MLMT score change of 2 was observed in the LUXTURNA treatment group, while a median MLMT score change of 0 was observed in the control group, when using both eyes or the first-treated eye. An MLMT score change of two or greater is considered a clinically meaningful benefit in functional vision.
Table 3 shows the number and percentage of subjects with different magnitudes of MLMT score change using both eyes at Year 1. Eleven of the 21 (52%) subjects in the LUXTURNA treatment group had an MLMT score change of two or greater, while one of the ten (10%) subjects in the control group had an MLMT score change of two.
Figure 6 shows MLMT performance of individual subjects using both eyes at Baseline and at Year 1.
Figure 6. MLMT Score Using Both Eyes at Baseline and Year 1 for Individual Subjects (Study 2)
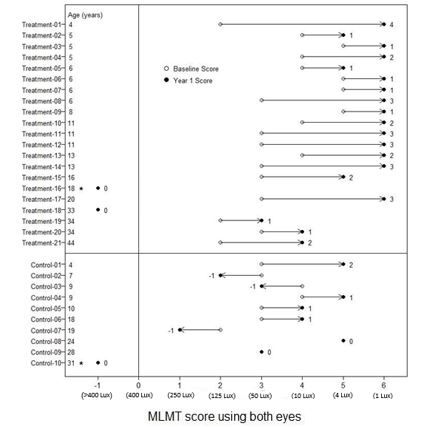
Note for Figure 6: *subjects who were withdrawn or discontinued. The open circles are the baseline scores. The closed circles are the Year 1 scores. The numbers next to the solid circle represent score change at Year 1. The horizontal lines with arrows represent the magnitude of the score change and its direction. Arrows pointing towards the right represent improvement. The top section shows the results of the 21 subjects in the treatment group. The bottom section shows the results of the 10 subjects in the control group. Subjects in each group are chronologically organized by age, with the youngest subject at the top and the oldest subject at the bottom.
Analysis of white light FST testing showed statistically significant improvement from Baseline to Year 1 in the LUXTURNA treatment group compared to the control group. The change in visual acuity from Baseline to Year 1 was not significantly different between the LUXTURNA and control groups.
Figure 7 shows the effect of LUXTURNA over the two-year period in the LUXTURNA treatment group, as well as the effect in the control group after crossing over to receive subretinal injection of LUXTURNA. A median MLMT score change of two was observed for the LUXTURNA treatment group at Day 30, and this effect was sustained over the remaining follow-up visits throughout the two-year period. For the control group, a median MLMT score change of 0 was observed at all four follow up visits during the first year. However, after crossing-over to receive subretinal injection of LUXTURNA, the subjects in the control group showed a similar response to LUXTURNA as compared to the subjects in the LUXTURNA treatment group.
Figure 7. MLMT Time-Course over Two Years: Using Both Eyes (Study 2)
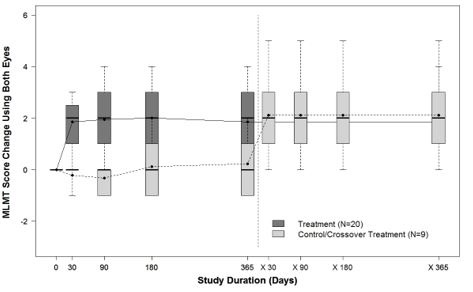
Note for Figure 7: Each box represents the middle 50% of distribution of MLMT score change. Vertical dotted lines represent additional 25% above and below the box. The horizontal bar within each box represents the median. The dot within each box represents the mean. The solid line connects the mean MLMT score changes over visits for the treatment group, including five visits during the first year and one visit at Year 2 (marked as x365). The dotted line connects the mean MLMT score change over visits for the control group, including five visits during the first year without receiving LUXTURNA, and four visits within the second year (marked as x30, x90, x180, and x365) after cross-over at Year 1 to receive LUXTURNA.
7HOW SUPPLIED/STORAGE AND HANDLING
Each carton of LUXTURNA (NDC 71394 – 415-01) contains one single-dose vial of the LUXTURNA (NDC 71394 – 065-01, 0.5 mL extractable volume) and two vials of Diluent (NDC 71394 – 716-01, 1.7 mL extractable volume in each vial). LUXTURNA contains 5 x 10
Store LUXTURNA and Diluent frozen at ≤ -65 °C.
Following thaw of the vials, store at room temperature. Store diluted LUXTURNA at room temperature [See Dosage and Administration 2.2].
LUXTURNA is an adeno-associated virus vector-based gene therapy. Follow universal biohazard precautions for handling.
8PATIENT COUNSELING INFORMATION
Advise patients and/or their caregivers of the following risks:
- Endophthalmitis and other eye infections
Serious infection can occur inside of the eye and may lead to blindness. In such cases, there is an urgent need for management without delay. Advise patients to call their healthcare provider if they experience new floaters, eye pain, or any change in vision.
- Permanent decline in visual acuity
Permanent decline in visual acuity may occur following subretinal injection of LUXTURNA. Advise patients to contact their healthcare provider if they experience any change in vision.
- Retinal abnormalities
Treatment with LUXTURNA may cause some defects in the retina such as a small tear or a hole in the area or vicinity of the injection. Treatment may cause thinning of the central retina, loss of retinal cells and the choroid (layer of blood vessels that line the back of the eye), or bleeding in the retina. Advise patients to follow up with their healthcare provider on a regular basis and report any symptoms such as decreased vision, blurred vision, flashes of light, or floaters in their vision without delay.
- Increased intraocular pressure
Treatment with LUXTURNA may cause transient or persistent increase in intraocular pressure. If untreated, such increases in intraocular pressure may cause blindness. Advise patients to follow-up with their healthcare provider to detect and treat any increase in intraocular pressure.
- Expansion of intraocular air bubbles
Advise patients to avoid air travel, travel to high elevations, or scuba diving until the air bubble formed following administration of LUXTURNA has completely dissipated from the eye. A change in altitude while the air bubble is still present may cause irreversible damage.
- Cataract
Advise patients that following treatment with LUXTURNA, they may develop a new cataract, or any existing cataract may get worse.
- Shedding of LUXTURNA
Transient and low level shedding of LUXTURNA may occur in patient tears. Advise patients and/or their caregivers on proper handling of waste material generated from dressing, tears, and nasal secretion, which may include storage of waste material in sealed bags prior to disposal. These handling precautions should be followed for up to 7 days following LUXTURNA administration.
Manufactured by:
US License #2056

