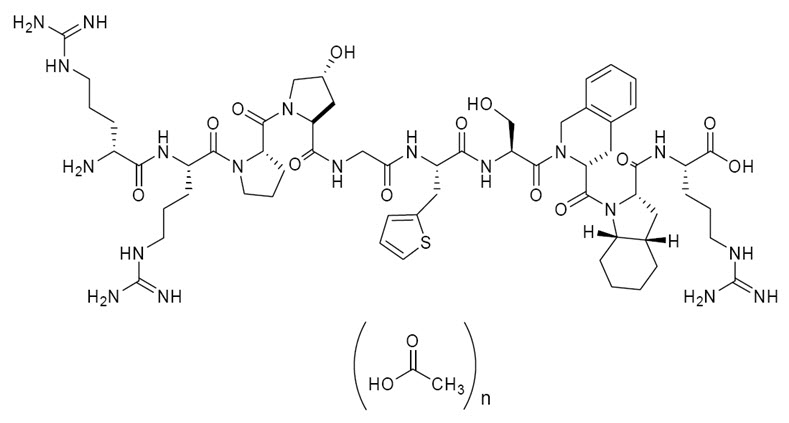
Icatibant
What is Firazyr (Icatibant)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Patients with HAE Type I or II who enroll in the study are asked to complete a patient diary when they experience an HAE attack. If icatibant is taken as the first treatment for the attack, the patient diary will ask questions over a 48 hour period after dosing to track the characteristics and severity of the attack along with the patient's level of anxiety.
Summary: Currently, there is no medication available to adequately treat patients undergoing hemodialysis who are suffering from intradialytic hypotension (IDH). Medical interventions such as Trendelenburg positioning, saline bolus administration, reduction of ultrafiltration rate, interruption of the hemodialysis, and other medical treatments are the methods of choice to treat the hypotensive condition of...
Summary: The goal of this project is to rapidly screen promising agents, in the setting of an adaptive platform trial, for treatment of critically ill COVID-19 patients. In this phase 2 platform design, agents will be identified with a signal suggesting a big impact on reducing mortality and the need for, as well as duration, of mechanical ventilation.
Related Latest Advances
Brand Information
One single-dose,
prefilled syringe and one 25G
hypodermic needle.
Full prescribing information with
patient injection instructions.
The syringe is closed with a protective cap.
FOR SUBCUTANEOUS USE ONLY

One single-dose,
prefilled syringe and one 25G
hypodermic needle.
Full prescribing information with
patient injection instructions.
The syringe is closed with a protective cap.
FOR SUBCUTANEOUS USE ONLY

Three cartons, each with one single-dose, prefilled syringe
and one 25G hypodermic needle.
Full prescribing information with patient injection instructions.
The syringe is closed with a protective cap.
FOR SUBCUTANEOUS USE ONLY