Brand Name
Nesina
Generic Name
Alogliptin
View Brand Information FDA approval date: January 25, 2013
Classification: Dipeptidyl Peptidase 4 Inhibitor
Form: Tablet
What is Nesina (Alogliptin)?
Alogliptin tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Alogliptin tablets are a dipeptidyl peptidase-4 inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Limitations of Use: Should not be used in patients with type 1 diabetes. Limitations of Use Alogliptin tablets should not be used in patients with type 1 diabetes mellitus.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Clinical Trial and Open-Label Extension Study to Evaluate the Efficacy and Safety of Add-On Therapy With Empagliflozin for Patients With Inadequately Controlled Type 2 DiabetesMellitus in the Combination Treatment With Metformin and Alogliptin
Summary: Phase 3 study to assess the Efficacy and Safety of CT-L02-301 in Type 2 Diabetes Patients with Insufficient Glycemic Control with Metformin and Alogliptin Combination Therapy.
Related Latest Advances
Brand Information
Nesina (alogliptin)
1INDICATIONS AND USAGE
NESINA
2DOSAGE FORMS AND STRENGTHS
- 25 mg tablets are light red, oval, biconvex, film-coated, with "TAK ALG-25" printed on one side.
- 12.5 mg tablets are yellow, oval, biconvex, film-coated, with "TAK ALG-12.5" printed on one side.
- 6.25 mg tablets are light pink, oval, biconvex, film-coated, with "TAK ALG-6.25" printed on one side.
3CONTRAINDICATIONS
NESINA is contraindicated in patients with a history of serious hypersensitivity to alogliptin or any of the excipients in NESINA. Reactions such as anaphylaxis, angioedema and severe cutaneous adverse reactions have been reported
4ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Pancreatitis
- Heart Failure
- Hypersensitivity Reactions
- Hepatic Effects
- Severe and Disabling Arthralgia
- Bullous Pemphigoid
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 14,778 patients with type 2 diabetes mellitus participated in 14 randomized, double-blind, controlled clinical trials of whom 9,052 subjects were treated with NESINA, 3,469 subjects were treated with placebo and 2,257 were treated with an active comparator. The racial distribution of patients exposed to trial medication was 71% White, 17% Asian, 6% Black or African American, 2% American Indian or Alaska Native, 0% Native Hawaiian/Other Pacific Islander and 5% Multiracial or other racial groups. The ethnic distribution was 30% Hispanic or Latino and 70% was not Hispanic or Latino. The mean duration of diabetes mellitus was seven years, the mean body mass index (BMI) was 31 kg/m
In a pooled analysis of these 14 controlled clinical trials, the overall incidence of adverse reactions was 73% in patients treated with NESINA 25 mg compared to 75% with placebo and 70% with active comparator. Overall discontinuation of therapy due to adverse reactions was 6.8% with NESINA 25 mg compared to 8.4% with placebo or 6.2% with active comparator.
Adverse reactions reported in ≥4% of adult patients treated with NESINA 25 mg and more frequently than in patients who received placebo are summarized in Table 1.
4.2Postmarketing Experience
The following adverse reactions have been identified during the postmarketing use of NESINA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: acute pancreatitis, diarrhea, constipation, nausea, ileus
Hepatobiliary Disorders: fulminant hepatic failure
Immune System Disorders: hypersensitivity reactions including anaphylaxis
Investigations: hepatic enzyme elevations
Musculoskeletal and Connective Tissue Disorders: severe and disabling arthralgia, rhabdomyolysis
Renal and Urinary Disorders: tubulointerstitial nephritis
Skin and Subcutaneous Tissue Disorders: angioedema, rash, urticaria and severe cutaneous adverse reactions including Stevens-Johnson syndrome, bullous pemphigoid
5OVERDOSAGE
In the event of an overdose, it is reasonable to institute the necessary clinical monitoring and supportive therapy as dictated by the patient's clinical status. Per clinical judgment, it may be reasonable to initiate removal of unabsorbed material from the gastrointestinal tract.
Alogliptin is minimally dialyzable; over a three-hour hemodialysis session, approximately 7% of the drug was removed. Therefore, hemodialysis is unlikely to be beneficial in an overdose situation. It is not known if NESINA is dialyzable by peritoneal dialysis.
In the event of an overdose, contact the Poison Help Line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
6DESCRIPTION
NESINA tablets contain the active ingredient alogliptin, which is a selective, orally bioavailable inhibitor of the enzymatic activity of DPP-4.
Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-({6-[(3
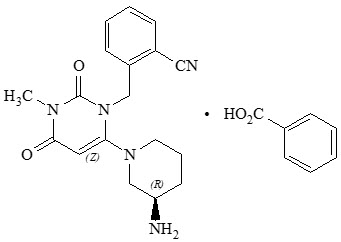
Alogliptin benzoate is a white to off-white crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol and very slightly soluble in octanol and isopropyl acetate.
Each NESINA tablet contains 34 mg, 17 mg or 8.5 mg alogliptin benzoate, which is equivalent to 25 mg, 12.5 mg or 6.25 mg, respectively, of alogliptin and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, mannitol, and microcrystalline cellulose. In addition, the film coating contains the following inactive ingredients: ferric oxide (red or yellow), hypromellose, polyethylene glycol, and titanium dioxide and is marked with printing ink (Gray F1).
7HOW SUPPLIED/STORAGE AND HANDLING
NESINA tablets are available as film-coated tablets containing 25 mg, 12.5 mg or 6.25 mg of alogliptin as follows:
25 mg tablet: light red, oval, biconvex, film-coated, with "TAK ALG-25" printed on one side, available in:
12.5 mg tablet: yellow, oval, biconvex, film-coated, with "TAK ALG-12.5" printed on one side, available in:
6.25 mg tablet: light pink, oval, biconvex, film-coated, with "TAK ALG-6.25" printed on one side, available in:
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
9PRINCIPAL DISPLAY PANEL - 6.25 mg Tablet Bottle Label
NDC 64764-625-30
Nesina®
(alogliptin) tablets
(alogliptin) tablets
6.25 mg
Dispense with
30 Tablets
Takeda
Rx Only

10PRINCIPAL DISPLAY PANEL - 12.5 mg Tablet Bottle Label
NDC 64764-125-30
Nesina®
(alogliptin) tablets
(alogliptin) tablets
12.5 mg
Dispense with
30 Tablets
Takeda
Rx Only

11PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle Label
NDC 64764-250-30
Nesina®
(alogliptin) tablets
(alogliptin) tablets
25 mg
Dispense with
30 Tablets
Takeda
Rx Only





