Bosulif
What is Bosulif (Bosutinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a long term safety study for patients who have completed a Novartis sponsored asciminib study and are judged by the investigator to benefit from continued treatment
Summary: Cancer drugs which target the effects of abnormal gene changes are called 'targeted therapies'. This study, called PM.1 or CAPTUR, will include some targeted therapies that are currently available. The purpose of this study is to find out what are the effects on a patient and their cancer when they are given a targeted therapy drug that is specific to an abnormal gene change in their cancer.
Summary: Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by the presence of the Philadelphia chromosome, resulting in the constitutive activation of the BCR-ABL1 tyrosine kinase. The advent of tyrosine kinase inhibitors (TKIs) has revolutionized the management of CML, trasforming it from a fatal disease to a chronic condition with excellent long-term outcomes for the majority ...
Related Latest Advances
Brand Information
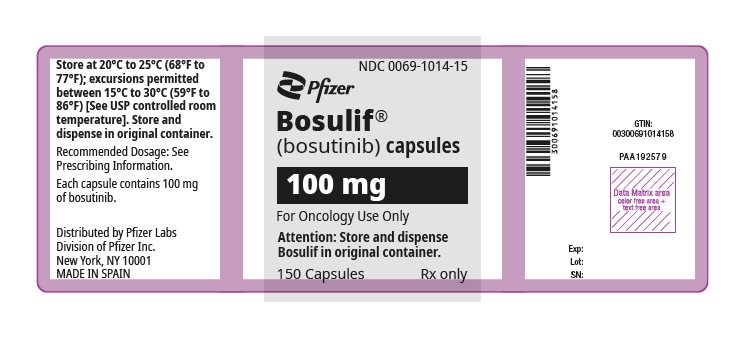
- Adult and pediatric patients 1 year of age and older with chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML), newly-diagnosed or resistant or intolerant to prior therapy
- Adult patients with accelerated phase (AP), or blast phase (BP) Ph+ CML with resistance or intolerance to prior therapy
- 100 mg: yellow, oval, biconvex, film-coated tablets debossed with "Pfizer" on one side and "100" on the other.
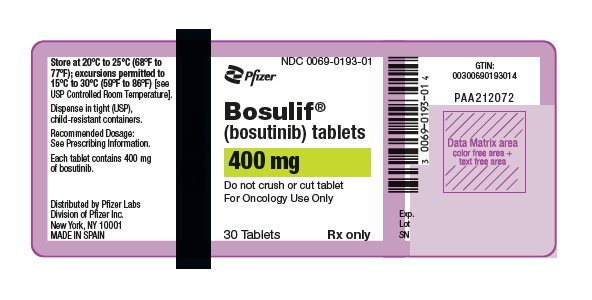
- 400 mg: orange, oval, biconvex, film-coated tablets debossed with "Pfizer" on one side and "400" on the other.
- 500 mg: red, oval, biconvex, film-coated tablets debossed with "Pfizer" on one side and "500" on the other.
- 50 mg: white body/orange cap with “BOS 50” printed on the body and “Pfizer” printed on the cap in black ink.
- 100 mg: white body/brownish-red cap with “BOS 100” printed on the body and “Pfizer” printed on the cap in black ink.
- Gastrointestinal toxicity
- Myelosuppression
- Hepatic toxicity
- Cardiovascular toxicity
- Fluid retention
- Renal toxicity

- BOSULIF capsules can be opened and the capsules contents mixed with room temperature applesauce or yogurt.
- Only use applesauce or yogurt.
- Swallow all of the mixture right away, without chewing.
- If you do not swallow the entire BOSULIF mixture, do not mix another dose. Wait until the next day to take your regularly scheduled dose.
- Take the BOSULIF mixture with a full meal.
Gather the following supplies:
- BOSULIF capsules
- small, clean container
- yogurt or applesauce
- teaspoon for mixing
- disposable gloves
- Store BOSULIF at room temperature between 68°F to 77°F (20°C to 25°C).
- Store the BOSULIF capsules in the original bottle.
- Ask your doctor or pharmacist about the right way to throw away outdated or unused BOSULIF.

(bosutinib) tablets
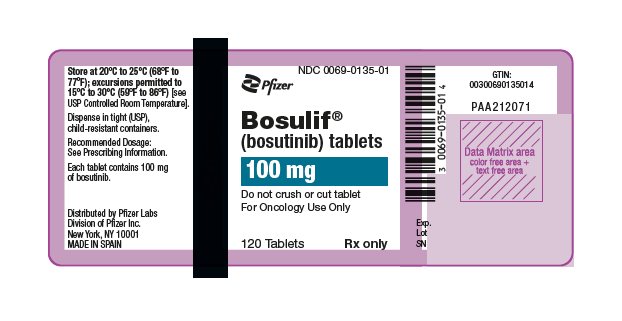
(bosutinib) tablets
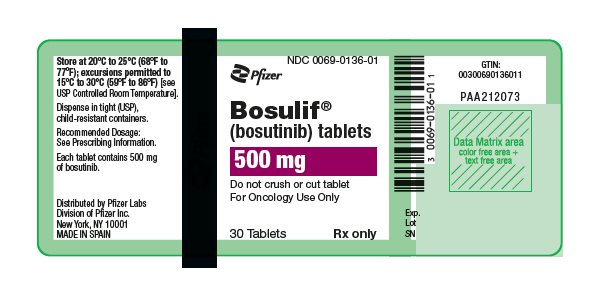
(bosutinib) tablets
(bosutinib) capsules
(bosutinib) capsules