The efficacy of ONPATTRO was demonstrated in a randomized, double-blind, placebo-controlled, multicenter clinical trial in adult patients with polyneuropathy caused by hATTR amyloidosis (NCT 01960348). Patients were randomized in a 2:1 ratio to receive ONPATTRO 0.3 mg/kg (N=148) or placebo (N=77), respectively, via intravenous infusion once every 3 weeks for 18 months. All patients received premedication with a corticosteroid, acetaminophen, and H1 and H2 blockers. Ninety-three percent of ONPATTRO-treated patients and 62% of placebo-treated patients completed 18 months of the assigned treatment.
The primary efficacy endpoint was the change from baseline to Month 18 in the modified Neuropathy Impairment Score +7 (mNIS+7). The mNIS+7 is an objective assessment of neuropathy and comprises the NIS and Modified +7 (+7) composite scores. In the version of the mNIS+7 used in the trial, the NIS objectively measures deficits in cranial nerve function, muscle strength, and reflexes, and the +7 assesses postural blood pressure, quantitative sensory testing, and peripheral nerve electrophysiology. The maximum possible score was 304 points, with higher scores representing a greater severity of disease.
The clinical meaningfulness of effects on the mNIS+7 was assessed by the change from baseline to Month 18 in Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) total score. The Norfolk QoL-DN scale is a patient-reported assessment that evaluates the subjective experience of neuropathy in the following domains: physical functioning/large fiber neuropathy, activities of daily living, symptoms, small fiber neuropathy, and autonomic neuropathy. The version of the Norfolk QoL-DN that was used in the trial had a total score range from -4 to 136, with higher scores representing greater impairment.
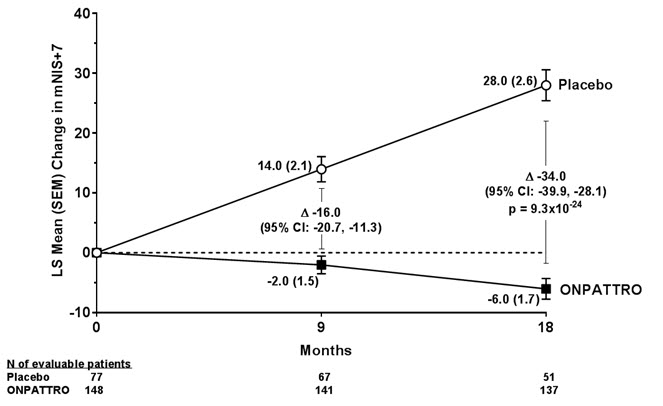
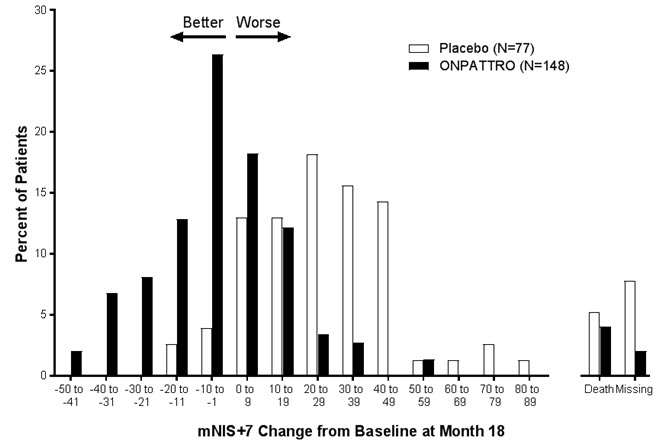
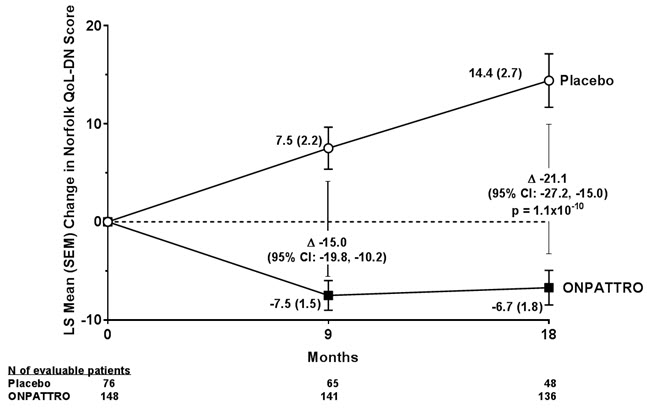
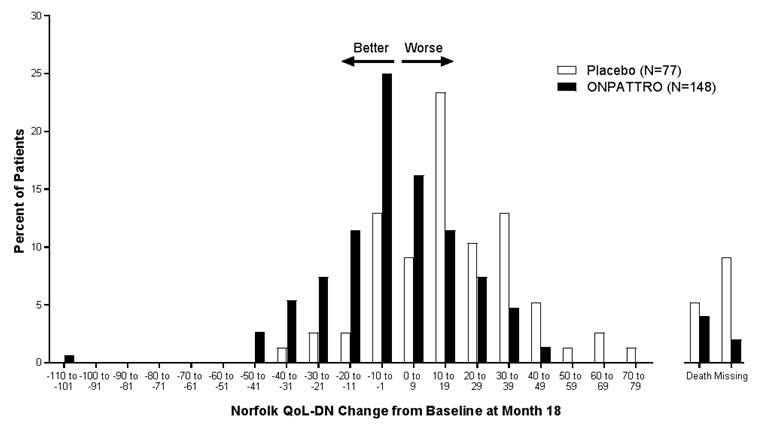
The changes from baseline to Month 18 on both the mNIS+7 and the Norfolk QoL-DN significantly favored ONPATTRO (Table 2, Figure 1 and Figure 3). The distributions of changes in mNIS+7 and Norfolk QoL-DN scores from baseline to Month 18 by percent of patients are shown in Figure 2 and Figure 4, respectively.
The changes from baseline to Month 18 in modified body mass index (mBMI) and gait speed (10-meter walk test) significantly favored ONPATTRO (Table 2).
Figure 1: Change from Baseline in mNIS+7
A decrease in mNIS+7 indicates improvement.
Δ indicates between-group treatment difference, shown as the LS mean difference (95% CI) for ONPATTRO – placebo.
Figure 2: Histogram of mNIS+7 Change from Baseline at Month 18
mNIS+7 change scores are rounded to the nearest whole number; last available post-baseline scores were used.
Categories are mutually exclusive; patients who died before 18 months are summarized in the "Death" category only.
Figure 3: Change from Baseline in Norfolk QoL-DN Score
A decrease in Norfolk QoL-DN score indicates improvement.
Δ indicates between-group treatment difference, shown as the LS mean difference (95% CI) for ONPATTRO – placebo.
Figure 4: Histogram of Norfolk QoL-DN Change from Baseline at Month 18
Norfolk QoL-DN change scores are rounded to the nearest whole number; last available post-baseline scores were used.
Categories are mutually exclusive; patients who died before 18 months are summarized in the "Death" category only.
Patients receiving ONPATTRO experienced similar improvements relative to placebo in mNIS+7 and Norfolk QoL-DN score across all subgroups including age, sex, race, region, NIS score, Val30Met mutation status, and disease stage.